filmov
tv
Predicting Single Replacement Reactions || Chemistry with Dr. G

Показать описание
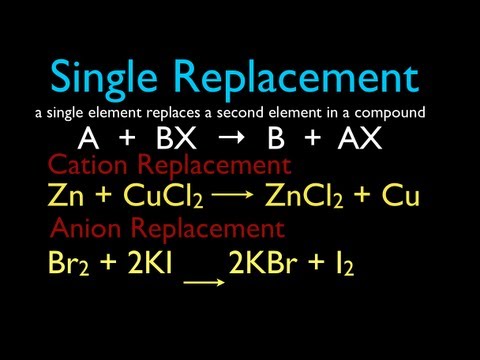
A single replacement reaction is a redox reaction in which one metal (or H) replaces another metal in a compound. These reactions do not always occur. In order for a single replacement reaction to occur, the more active metal must be the one replacing the lesser active metal that is in the compound. An example is shown below.
Mg(s) + ZnCl2(aq) → MgCl2(aq) + Zn(s)
The Mg is the more active metal as it can replace Zn2+ in ZnCl2.
Mg(s) + ZnCl2(aq) → MgCl2(aq) + Zn(s)
The Mg is the more active metal as it can replace Zn2+ in ZnCl2.
 0:02:03
0:02:03
 0:10:18
0:10:18
 0:06:28
0:06:28
 0:05:59
0:05:59
 0:14:05
0:14:05
 0:06:38
0:06:38
 0:05:39
0:05:39
 0:09:28
0:09:28
 0:06:32
0:06:32
 0:08:28
0:08:28
 0:05:35
0:05:35
 0:13:38
0:13:38
 0:06:24
0:06:24
 0:09:24
0:09:24
 0:10:37
0:10:37
 0:07:32
0:07:32
 0:08:59
0:08:59
 0:12:54
0:12:54
 0:10:02
0:10:02
 0:13:03
0:13:03
 0:11:17
0:11:17
 0:03:00
0:03:00
 0:25:19
0:25:19
 0:04:10
0:04:10