filmov
tv
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL)

Показать описание
This video covers the calculation of ∆H for reactions using ∆H°f data.
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL)
R1.2.3/5.1 Standard Enthalpy Change of Formation and Combustion [HL IB Chemistry 2024]
R1.2.3 / R1.2.4 Standard enthalpy change of combustion (HL)
2020-02-22 APC WDSF Open Senior IV Standard R1 Slowfox Heat 3
Calculate Enthalpy, Enthropy Change for Reaction
Enthalpy Changes [IB Chemistry SL/HL]
⚗️ Change in Enthalpy and Standard Enthalpies of Formation (Part 1)
Calculating the enthalpy change of a reaction
How to read P h Chart explained with Numerical
KAC 13.14 - Enthalpy I: Hess's Law - Use of Enthalpies of Combustion
Standard enthalpy change for combustion | Thermodynamics | Chemistry | Khan Academy
Hess's Law - Chemistry Tutorial
R1.2.5 Born-Haber cycles (HL)
CHEM 101 - Using Standard Enthalpies of Formation and Standard Enthalpy Change
34.4 standard enthalpy change of formation
15.2/R1.4 Calculate the standard entropy change for a reaction [HL IB Chemistry]
Calculate Enthalpy of Reaction (∆Hrxn) By Reaction Addition Method 001
Enthalpy Changes IAL As Chemistry Unit 2 Lecture 3
Road VS Track TIRE PRESSURES (Sous Titres 🇫🇷)
Bond Enthalpy Calculations (IB Chemistry R1.2)
Enthalpy of combustion
Calculate Change in Enthalpy (using Bond Energy)
3.1.4 - Energetics Lesson 3 of 3 - Bond Enthalpy
Calculating the enthalpy change of a reaction
Комментарии
 0:02:53
0:02:53
 0:08:00
0:08:00
 0:03:07
0:03:07
 0:01:46
0:01:46
 0:07:17
0:07:17
 0:11:56
0:11:56
 0:04:17
0:04:17
 0:08:24
0:08:24
 0:10:13
0:10:13
 0:10:07
0:10:07
 0:07:22
0:07:22
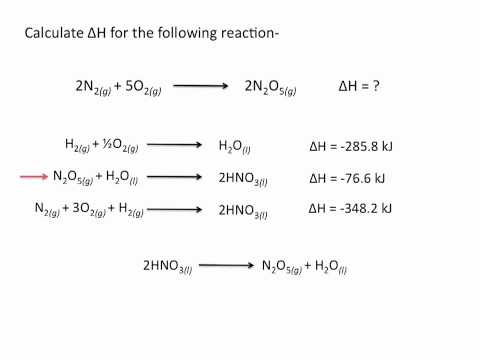 0:11:23
0:11:23
 0:03:32
0:03:32
 0:02:36
0:02:36
 0:15:13
0:15:13
 0:02:45
0:02:45
 0:05:11
0:05:11
 0:24:34
0:24:34
 0:02:20
0:02:20
 0:10:18
0:10:18
 0:05:31
0:05:31
 0:04:45
0:04:45
 0:21:49
0:21:49
 0:09:48
0:09:48