filmov
tv
15.2/R1.4 Calculate the standard entropy change for a reaction [HL IB Chemistry]
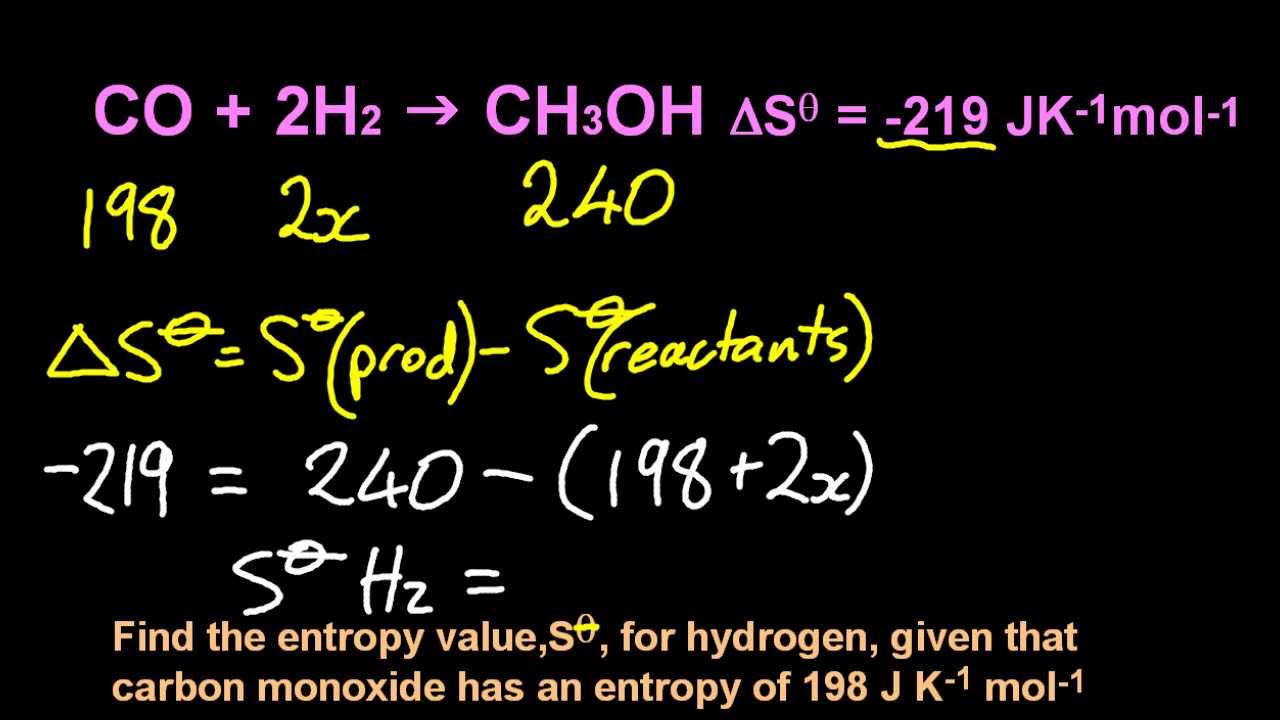
Показать описание
15.2 Calculate the standard entropy change for a reaction using standard entropy values. Same old same old -- this is just products minus reactants. Don't forget to account for the coefficients. The chemicals themselves have ENTROPY values ,S, but the equation has the CHANGE IN ENTROPY value, delta S.
Calculate Enthalpy, Enthropy Change for Reaction
Calculate Enthalpy, Enthropy Change for Reaction
Calculate the standard entropy change for the following reaction at 25 °C
Estimating and calculating reaction entropy using standard entropies (19.52)
Calculation of Standard Molar Entropy
Calculate standard entropy change of reaction
calculating entropy
15.3.1 -15.3.2 State and explain the factors that increase the entropy in a system (HL)
9: Calculating Entropy
Calculate Reaction Change in Enthalpy, Entropy and Free Energy
IB Chemistry Topic 15.2 (HL): Entropy & Spontaneity
Calculate Reaction Change in Enthalpy, Entropy and Free Energy
What is Entropy? (IB Chemistry R1.4)
Standard Reaction Entropy Change problem example
IQ TEST
Salsa Night in IIT Bombay #shorts #salsa #dance #iit #iitbombay #motivation #trending #viral #jee
Standard molar entropies
Standard Entropy and Free Energy | General Chemistry II | 4.1
Find Entropy of Rxn from Standard Entropies
Standard Molar Entropy and the Third Law
IB Chemistry Topic 5 Energetics HL 15.2 Entropy and spontaneity
How to Calculate ∆G° Standard Gibb's Free Energy Change of a Reaction from Enthalpy H and Entro...
Standard Molar Entropy
IB FRQ 15 Thermochemistry
Комментарии
 0:07:17
0:07:17
 0:07:17
0:07:17
 0:12:24
0:12:24
 0:04:06
0:04:06
 0:06:29
0:06:29
 0:05:35
0:05:35
 0:10:32
0:10:32
 0:03:24
0:03:24
 0:02:29
0:02:29
 0:11:32
0:11:32
 0:04:59
0:04:59
 0:11:32
0:11:32
 0:10:30
0:10:30
 0:05:15
0:05:15
 0:00:29
0:00:29
 0:00:14
0:00:14
 0:15:45
0:15:45
 0:15:40
0:15:40
 0:09:39
0:09:39
 0:05:36
0:05:36
 0:08:47
0:08:47
 0:04:05
0:04:05
 0:04:10
0:04:10
 0:15:19
0:15:19