filmov
tv
The Reaction Quotient

Показать описание
063 - The Reaction Quotient
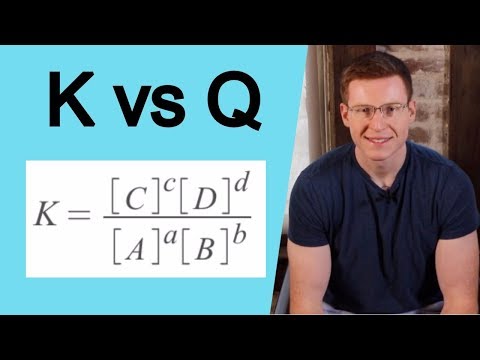
In this video Paul Andersen explains how the reaction quotient is used to determine the progress of a reversible reaction. The reaction quotient (Q) is the ratio of the concentration of products to the concentration of reactants. The reaction quotient will equal the equilibrium constant when the reaction is at equilibrium. Model and graphical analysis of Q is included.
Do you speak another language? Help me translate my videos:
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
In this video Paul Andersen explains how the reaction quotient is used to determine the progress of a reversible reaction. The reaction quotient (Q) is the ratio of the concentration of products to the concentration of reactants. The reaction quotient will equal the equilibrium constant when the reaction is at equilibrium. Model and graphical analysis of Q is included.
Do you speak another language? Help me translate my videos:
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
Chemical Equilibria and Reaction Quotients
K (Equilibrium Constant) vs Q (Reaction Quotient)
Introduction to reaction quotient Qc | Chemical equilibrium | Chemistry | Khan Academy
The Reaction Quotient
Using the reaction quotient | Equilibrium | AP Chemistry | Khan Academy
Reaction Quotient (K) and Equilibrium Constant (K) Problems & Examples. Which way the reaction s...
Q Reaction Quotient
Writing equilibrium constant and reaction quotient expressions | AP Chemistry | Khan Academy
Your TI-84 Chemistry Formula Program | Ace Your Chem Exams in Record Time! #chemistry #ti84programs
Reaction Quotient - Explained
Writing the Reaction Quotient (Qc) From the Unbalanced Equation 001
14.3 The Reaction Quotient
Equilibrium Constant (K) & Reaction Quotient (Q)
Introduction to the Reaction Quotient
15.2 Le Chatelier's Principle | General Chemistry
Writing the Reaction Quotient (Qc) From the Balanced Equation 002
10: Reaction quotient (Q) vs. equilibrium constant (K)
Chemical Equilibrium | Reaction Quotient & Application of a Large K.
Worked example: Using the reaction quotient to predict a pressure change | Khan Academy
Reaction Quotient
Reaction Quotients
What is a Reaction Quotient, and How do I use it?
Worked example: Using the reaction quotient to find equilibrium partial pressures | Khan Academy
The Reaction Quotient, Q
Комментарии
 0:06:48
0:06:48
 0:03:00
0:03:00
 0:07:36
0:07:36
 0:07:08
0:07:08
 0:07:50
0:07:50
 0:06:15
0:06:15
 0:08:43
0:08:43
 0:07:03
0:07:03
 0:17:28
0:17:28
 0:14:44
0:14:44
 0:01:48
0:01:48
 0:09:03
0:09:03
 0:06:37
0:06:37
 0:00:19
0:00:19
 0:25:12
0:25:12
 0:01:51
0:01:51
 0:05:52
0:05:52
 0:06:45
0:06:45
 0:05:53
0:05:53
 0:08:59
0:08:59
 0:04:23
0:04:23
 0:06:02
0:06:02
 0:06:31
0:06:31
 0:03:35
0:03:35