filmov
tv
Phase Transitions: Sublimation and Deposition | Chem | Video Textbooks - Preview

Показать описание
JoVE is the world-leading producer and provider of science videos with a mission to accelerate scientific research and education. Millions of scientists, educators and students across 1500+ High Schools, colleges and universities worldwide use JoVE’s library of 17,000+ videos for teaching, learning and research.
JoVE High Schools offers expert-created video textbooks, lab videos, science experiment videos, interactive assessments, and seamless integration into school systems. These comprehensive learning resources help educators tackle teaching challenges, engage students, and meet curriculum requirements, enhancing the high school learning experience.
Follow us on -
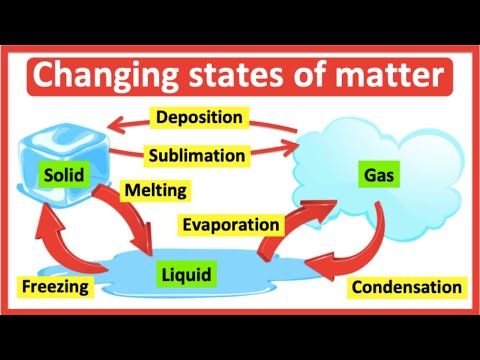
It is commonly observed that regular ice melts under ambient conditions, but dry ice does not; instead, dry ice transitions directly into the gas phase. This transition from solid to gas — without passing through the liquid phase — is known as sublimation.
Generally, compounds that sublimate exhibit weak intermolecular forces in the solid state.
 0:00:23
0:00:23
 0:01:25
0:01:25
 0:04:28
0:04:28
 0:02:08
0:02:08
 0:04:01
0:04:01
 0:02:46
0:02:46
 0:03:10
0:03:10
 0:18:51
0:18:51
 0:08:26
0:08:26
 0:02:23
0:02:23
 0:01:50
0:01:50
 0:01:39
0:01:39
 0:04:51
0:04:51
 0:01:52
0:01:52
 0:05:24
0:05:24
 0:11:52
0:11:52
 0:05:05
0:05:05
 0:02:21
0:02:21
 0:08:29
0:08:29
 0:02:47
0:02:47
 0:06:21
0:06:21
 0:04:36
0:04:36
 0:02:29
0:02:29
 0:00:54
0:00:54