filmov
tv
OCR A 5.3.1 and 5.3.2 Transition Elements and Qualitative Analysis REVISION

Показать описание
OCR Pure Core: Complex numbers 5-3
OCR Pure Core: Vectors 5-3
OCR Pure Core: Vectors 5-3
OCR MEI Core 1 6.05 Describe the Transformation that maps y = (x - 4)^2 + 5 onto y = (x + 3)^2 - 2
OCR Pure Core: Complex numbers 3-5
OCR A 5.3.1 and 5.3.2 Transition Elements and Qualitative Analysis REVISION
LA SORPRESA
How to calculate Negative Indices Fractions? #math #tutor #fraction #indices #power #exponents #x^-2
NEWYES Calculator VS Casio calculator
OCR Pure Core: Complex numbers 5-2
OCR Pure Core: Complex numbers 3-1
OCR Pure Core: Complex numbers 5-4
OCR MEI Core 1 4.05 Solving (x + 5)(x + 3) less than 0
OCR A LEVEL CHEMISTRY 2021 ALL 3 PAPERS
Comparing Fractions | How to Compare Fractions
Area of a Triangle With Vertices - Geometry
OCR A 5.1.3 Acids, Bases and Buffers REVISION
OCR Pure Core: Vectors 5-2
Cool gadgets!😎Smart appliances, Home items/utensils for the kitchen [Makeup&Beauty]#shorts
DNA Replication (Updated)
OCR MEI Core 3 4.03 Using the Chain Rule to Differentiate y = (3x - 5)^6
OCR MEI Core 1 8.06 Binomial Expansion: Fully Expand (x + 5/x)^5
Writing an equation of circle tangent to the x axis given the center
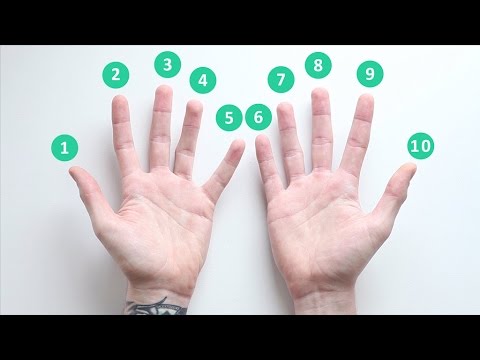
Nine times table | Multiplication hand trick
Комментарии
 0:08:44
0:08:44
 0:09:09
0:09:09
 0:07:03
0:07:03
 0:02:10
0:02:10
 0:06:15
0:06:15
 1:02:43
1:02:43
 0:00:31
0:00:31
 0:00:36
0:00:36
 0:00:14
0:00:14
 0:06:02
0:06:02
 0:11:05
0:11:05
 0:07:28
0:07:28
 0:00:59
0:00:59
 0:00:56
0:00:56
 0:07:43
0:07:43
 0:05:06
0:05:06
 1:22:25
1:22:25
 0:05:30
0:05:30
 0:00:34
0:00:34
 0:08:12
0:08:12
 0:03:27
0:03:27
 0:04:42
0:04:42
 0:01:29
0:01:29
 0:03:05
0:03:05