filmov
tv
Writing an Equilibrium Expression (Mass Action Equation)

Показать описание
This short video shows you how to write an equilibrium expression. I discuss what the constant is used for. I discuss which phases to include in this setup. I also go over a examples.
Example reactions:
3 O2(g) ↔ 2 O3(g)
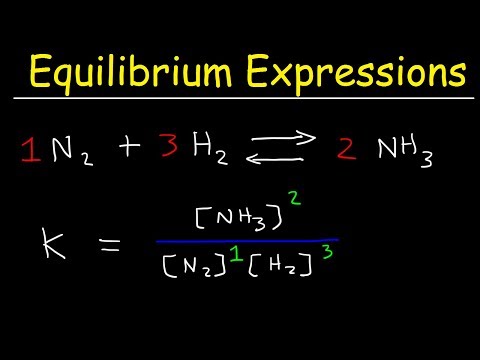
N2(g) + 3 H2(g) ↔ 2 NH3(g)
Cu(s) +2Ag+(aq) ↔ Cu2+(aq) +2Ag(s)
CaCO3(s) ↔ CaO(s) + O2(g)
Example reactions:
3 O2(g) ↔ 2 O3(g)
N2(g) + 3 H2(g) ↔ 2 NH3(g)
Cu(s) +2Ag+(aq) ↔ Cu2+(aq) +2Ag(s)
CaCO3(s) ↔ CaO(s) + O2(g)
How To Write The Equilibrium Expression For a Chemical Reaction - Law of Mass Action
Writing an Equilibrium Expression (Mass Action Equation)
Writing Equilibrium Expressions
How to write the equilibrium expression (Kc): 3 Trick Questions
Write an equilibrium expression from a chemical equation
How to Write Equilibrium Constant Expression (K, Keq, Kc, Kp) Practice Problems, Examples, Summary
Equilibrium Expressions and K
Writing an Equilibrium Expression
Physics 12 | Mr. Wrya Jaleel | Chapter 3 | Lesson #10
Writing an Equilibrium Expression 1
Chemical Equilibria and Reaction Quotients
ALEKS: Writing the concentration equilibrium expression for a heterogeneous equilibrium
Writing Equilibrium Expressions
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
Derivation of Equilibrium Expression
How to Write Equilibrium Expressions (Keq) 2017
How to Write an Equilibrium Expression
16 2 writing equilibrium expressions
How to Write Equilibrium Constant Expressions | Kc Keq Kp | Basics Chemical Equilibrium
11: How to write equilibrium expressions
Writing equilibrium constant and reaction quotient expressions | AP Chemistry | Khan Academy
How to Write the Equilibrium Constant Expression for a Reaction (Chemical Equilibrium).
Equilbrium Constant Expression Kp
Equilibrium Expression for Homogeneous vs. Heterogeneous Equilibria
Комментарии
 0:05:24
0:05:24
 0:02:59
0:02:59
 0:10:20
0:10:20
 0:04:32
0:04:32
 0:00:57
0:00:57
 0:04:47
0:04:47
 0:05:23
0:05:23
 0:04:57
0:04:57
 0:40:35
0:40:35
 0:01:49
0:01:49
 0:06:48
0:06:48
 0:01:29
0:01:29
 0:14:47
0:14:47
 0:53:22
0:53:22
 0:04:21
0:04:21
 0:04:46
0:04:46
 0:01:40
0:01:40
 0:03:25
0:03:25
 0:07:31
0:07:31
 0:07:57
0:07:57
 0:07:03
0:07:03
 0:07:26
0:07:26
 0:03:04
0:03:04
 0:04:46
0:04:46