filmov
tv
Proton NMR - The Basics

Показать описание
Watch this video to find out the D2O shake! The first stop when it comes to finding out how proton NMR works. This video will look at how you can spot hydrogen environments and link them with a spectra.
Proton NMR - The Basics
NMR Spectroscopy
How to Identify Molecules - Proton NMR: Crash Course Organic Chemistry #26
Basic Introduction to NMR Spectroscopy
NMR spectroscopy visualized
Introduction to proton NMR | Spectroscopy | Organic chemistry | Khan Academy
NMR Spectroscopy for Visual Learners
Everything You Need To Know About NMR Spectra | MCAT Content
Proton NMR basics
Proton NMR Spectroscopy - How To Draw The Structure Given The Spectrum
Proton NMR - How To Analyze The Peaks Of H-NMR Spectroscopy
Integration of H NMR Signals - Spectroscopy - Organic Chemistry
How To Draw The Proton NMR Spectrum of an Organic Molecule
1D 1H-NMR in Mnova - Getting started
Proton NMR Spectroscopy: What You Need to Know // HSC Chemistry
NMR Spectroscopy: Basic Theory
NMR Part 1: The Instrument Basics
1H NMR basics Organic Spectroscopy 1
H-NMR Spectroscopy Basics [Livestream Recording] Organic Chemistry Review & Practice Session
NMR Spectroscopy Introduction | Lab Instrumentation and Principle
Proton NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) Made Easy // HSC Chemistry
Basics of 1H-NMR spectroscopy Part 1- 1H-NMR spectrum for structure prediction #1HNMR #NMRspectrum
Proton NMR 1 - Basic Spectra
Комментарии
 0:11:25
0:11:25
 0:14:36
0:14:36
 0:11:27
0:11:27
 0:11:40
0:11:40
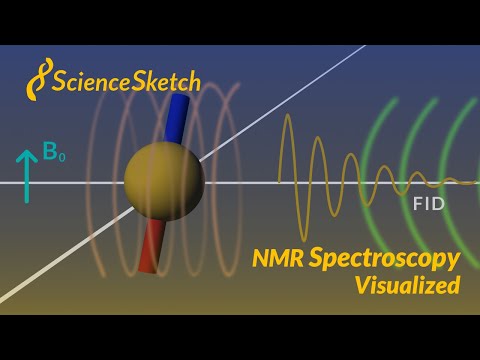 0:06:49
0:06:49
 0:10:27
0:10:27
 0:23:55
0:23:55
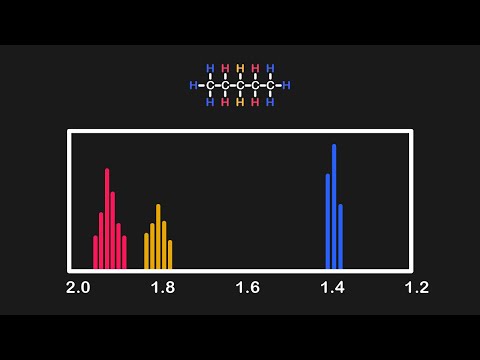 0:11:18
0:11:18
 0:56:45
0:56:45
 0:14:12
0:14:12
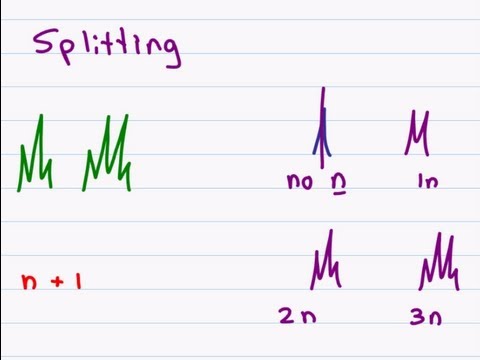 0:11:31
0:11:31
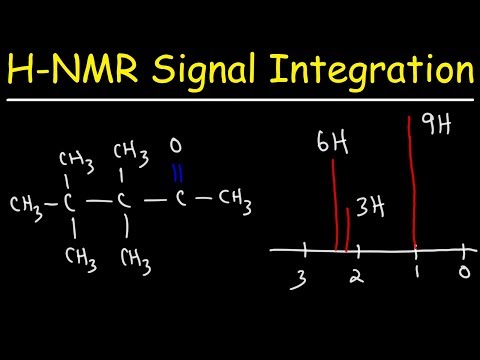 0:05:29
0:05:29
 0:12:06
0:12:06
 0:03:35
0:03:35
 0:07:38
0:07:38
 0:11:14
0:11:14
 0:24:23
0:24:23
 0:17:27
0:17:27
 0:58:07
0:58:07
 0:18:49
0:18:49
 0:14:15
0:14:15
 0:07:48
0:07:48
 0:09:59
0:09:59
 0:10:05
0:10:05