filmov
tv
NTA Blunder in JEE Main 2023 April Attempt🤦♂️😱 #jee2023 #iitjee #jeecutoff #esaral

Показать описание
📞𝐅𝐨𝐫 𝐦𝐨𝐫𝐞 𝐝𝐞𝐭𝐚𝐢𝐥𝐬, 𝐜𝐨𝐧𝐭𝐚𝐜𝐭 𝐡𝐞𝐫𝐞: +91-6376440597, +91-9024464479
▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬
🔴 Subscribe to Our Other Channels -
▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬
🔵 Join Our Telegram Channels -
▬▬▬▬▬▬▬▬▬▬▬▬▬▬▬
😱NTA BLUNDER Mistake | JEE Mains 2024🤬
NTA Blunder: Record Low Registrations for JEE Main 2025 #xandyjee #jee2025 #jeeapplication
😱NTA BIGGEST Mistake | JEE Mains 3rd Attempt 2022🤬
NTA Blunder in JEE Main 2023 April Attempt🤦♂️😱 #jee2023 #iitjee #jeecutoff #esaral
NTA Blunder: Lowest Registration in History in JEE Main 2025 #jee1 #jee2025
NTA- UNFAIR Result #jee2024
JEE Main 2021: Big Blunder by NTA | Learning for all of you ...
BIG BLUNDER IN JEE 2025 PREPARATION 🥵🥵 ? #jee #jeemains #jee2025 #jee2025preparation #nta
NTA Blunders-JEE MAIN 2022- TIME FOR ACTION😡
JEE Main 2022 Cheating Exposed 🤯 Blunder Results by NTA ! #jee #nta #jeemain2022
NV Sir Request to NTA after JEE Main 2024 Result | #kota #nvsir #nta #jee
NTA Update✅ Rejecting Application Form JEE Mains 2025 ⚠️ | JEE Main form fill up 2025 #jee2025
Shocking 🤯 JEE Main 2022 Cheating Exposed Blunder Results by NTA ! #jee #nta #jeemain2022 #shorts
Request to NTA: Blunders in JEE Main 2022 | July Attempt - ATP STAR Kota
🤣NTA funny video! JEE Mains 2024 results🤣🔥 #jee #iit #shorts
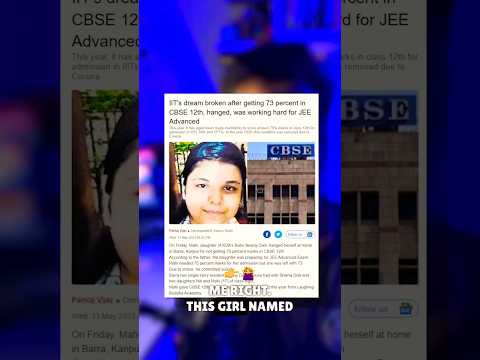
NTA killed this girl 😡😭 75% Criteria of NTA should be removed #jee
Big Blunder by NTA - JEE Main 2022 | ATP STAR KOTA
🥶Big Blunder by JEE Main 2021 Aspirants #shorts #NTA
Diversion from NTA's Biggest Blunder of 2022 ? 🤔 #shorts #eduniti
🤯Colleges without 75% criteria ft. @JEEVedantu ! NTA vs NCPCR | IIT Motivation | JEE Mains 2023
🙏Request to NTA - Big Blunder in answer key | JEE Main 2022 | ATP STAR KOTA
NTA blunder : JEE Mains 2021 July - Official answer key corrections | Vineet Khatri
NTA Finally fixing Blunder | JEE 2025 Registration #jee2025 #jee1
Most SHOCKING Marks vs Percentile 😭😱😡| JEE Mains Result 2024 #shorts #esaral #jee2024 #jeemains...
Комментарии
 0:00:27
0:00:27
 0:03:35
0:03:35
 0:00:12
0:00:12
 0:01:00
0:01:00
 0:06:50
0:06:50
 0:01:00
0:01:00
 0:00:53
0:00:53
 0:01:00
0:01:00
 0:02:33
0:02:33
 0:09:43
0:09:43
 0:01:00
0:01:00
 0:03:35
0:03:35
 0:00:56
0:00:56
 0:02:38
0:02:38
 0:00:15
0:00:15
 0:00:44
0:00:44
 0:07:39
0:07:39
 0:03:18
0:03:18
 0:01:00
0:01:00
 0:00:32
0:00:32
 0:04:03
0:04:03
 0:00:40
0:00:40
 0:08:56
0:08:56
 0:00:31
0:00:31