filmov
tv
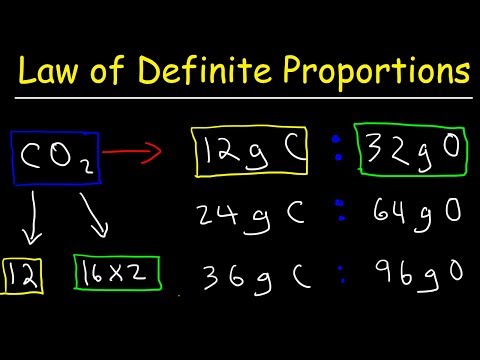
⚗️ Law of Definite Proportions

Показать описание
Q1. Two samples of carbon dioxide are decomposed into their constituent elements. One sample produces 25.6 g of oxygen and 9.60 g of carbon, and the other produces 21.6 g of oxygen and 8.10 g of carbon. Show that these results are consistent with the law of definite proportions.
Q2. Two samples of carbon monoxide are decomposed into their constituent elements. One sample produces 17.2 g of oxygen and 12.9 g of carbon, and the other sample produces 10.5 g of oxygen and 7.88 g of carbon. Show that these results are consistent with the law of definite proportions.
Law of Definite Proportions Chemistry Practice Problems - Chemical Fundamental Laws
Law of definite proportions | Atoms and Molecules | Chemistry | Khan Academy
Law of Definite Proportions
Law of Definite Proportions
Law of Constant Proportions | Don't Memorise
The Creation of Chemistry - The Fundamental Laws: Crash Course Chemistry #3
Chemistry 5.8a Law of Definite Proportions
law of definite proportion
Law of definite proportion #Ratio by mass# Chemistry #
Law Of Constant Composition | Properties of Matter | Chemistry | FuseSchool
Law of Multiple Proportions Practice Problems, Chemistry Examples, Fundamental Chemical Laws
The Law of Definite Proportions
Law of Definite Proportions
⚗️ Law of Definite Proportions
Example 2.1 Law of Definite Proportions
law of definite proportion | arvind arora chemistry class 11 | a2motivation
Proust's Law (Law of Definite Proportions)
law of definite proportions Practice Problems
Law of Definite Composition
Laws of Chemical Combinations - Class 9 Tutorial
Law of Definite Proportion #physicswallah
Laws of Chemical Combinations
Law of Definite Proportion
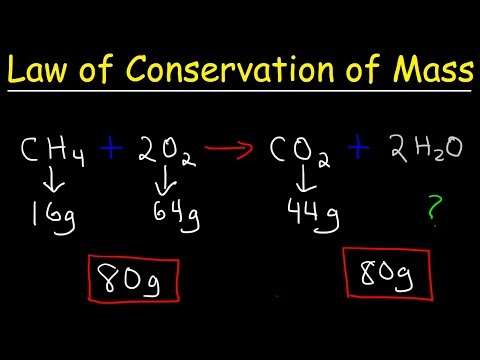
Law of Conservation of Mass - Fundamental Chemical Laws, Chemistry
Комментарии
 0:10:31
0:10:31
 0:02:19
0:02:19
 0:03:54
0:03:54
 0:02:41
0:02:41
 0:03:23
0:03:23
 0:10:59
0:10:59
 0:01:45
0:01:45
 0:00:55
0:00:55
 0:00:46
0:00:46
 0:03:48
0:03:48
 0:11:42
0:11:42
 0:09:42
0:09:42
 0:02:36
0:02:36
 0:03:22
0:03:22
 0:03:29
0:03:29
 0:02:26
0:02:26
 0:07:50
0:07:50
 0:03:25
0:03:25
 0:11:12
0:11:12
 0:05:14
0:05:14
 0:01:03
0:01:03
 0:15:48
0:15:48
 0:00:56
0:00:56
 0:03:14
0:03:14