filmov
tv
1-8. Determine the mass of the oxygen in the tank (Chapter 1: Fluid Mechanics Hibbeler)

Показать описание
SUBSCRIBE my Channel Engineers Academy for more problem Solutions!
Kindly like, share and comment, this will help to promote my channel!!
Fluid Mechanics by R.C. Hibbeler
Chapter 1 - Fundamental Concepts
Basic Fluid Properties
1-8. The bottle tank has a volume of 0.12m^3 and contains oxygen at an absolute pressure of 12MPa and a temperature of 30 degree C. Determine the mass of oxygen in the tank.
Fluid Mechanics by R.C. Hibbeler Solution
Kindly like, share and comment, this will help to promote my channel!!
Fluid Mechanics by R.C. Hibbeler
Chapter 1 - Fundamental Concepts
Basic Fluid Properties
1-8. The bottle tank has a volume of 0.12m^3 and contains oxygen at an absolute pressure of 12MPa and a temperature of 30 degree C. Determine the mass of oxygen in the tank.
Fluid Mechanics by R.C. Hibbeler Solution
Determining Mass from Density and Volume
Measuring Mass with a Scale
How to find density, mass, and volume
Atomic Number, Mass Number, and Net Electric Charge
Calculating Mass and Weight
Calculate %m/m (Percent by Mass of a solution)
Relative Formula Mass - mole concept
Easy trick to learn || Atomic mass|| 1 to 30 elements
#Grade 6 #KNEC #Mathematics KPSEA Preparation: Solved Question by Question
How To Calculate The Average Atomic Mass
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
How To Calculate The Molar Mass of a Compound - Quick & Easy!
Atomic Mass Unit | Chemistry
Atomic Number and Mass Number.mov
Hydrogen and oxygen combine in the ratio of 1:8 by mass to form water. What mass of oxygen gas w...
How to calculate molecular mass and molecular weight?
Force, Mass, and Acceleration: Newton's Second Law
Difference between MASS and WEIGHT
3.21 | Determine the mass in grams of each of the following: (a) 0.600 mol of oxygen atoms (b) 0.600
3.19 | Determine the mass of each of the following: (a) 2.345 mol LiCl (b) 0.0872 mol acetylene
How to calculate molecular mass/molecular weight
The law of conservation of mass - Todd Ramsey
Formula Mass and Molar Mass of a Compound
Calculate the mass of water produced when 8.25 grams of butane reacts with excess oxygen.
Комментарии
 0:01:18
0:01:18
 0:01:48
0:01:48
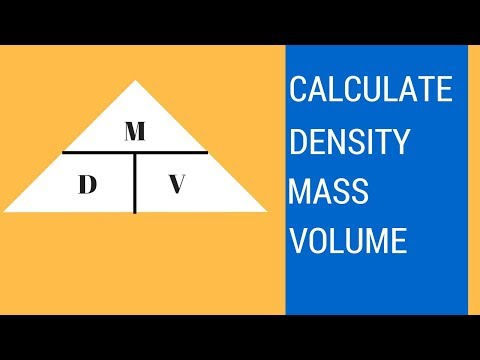 0:05:17
0:05:17
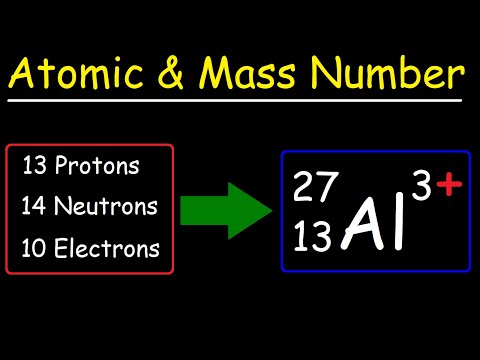 0:11:41
0:11:41
 0:04:26
0:04:26
 0:04:30
0:04:30
 0:05:57
0:05:57
 0:07:04
0:07:04
 0:40:01
0:40:01
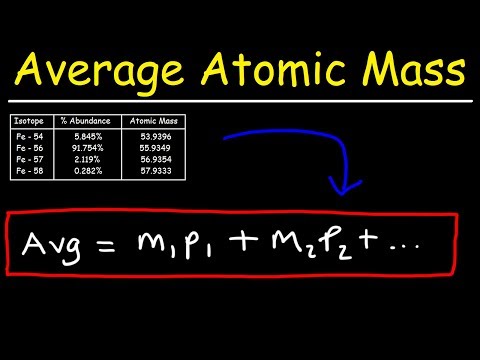 0:07:19
0:07:19
 0:31:25
0:31:25
 0:11:20
0:11:20
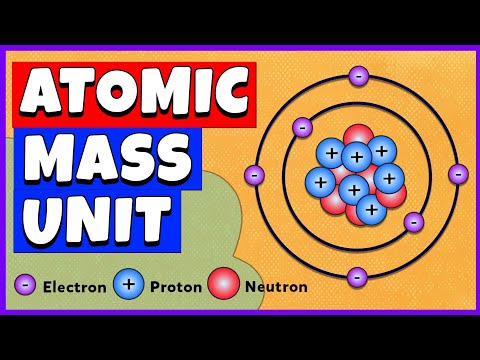 0:11:53
0:11:53
 0:06:13
0:06:13
 0:02:32
0:02:32
 0:04:50
0:04:50
 0:02:03
0:02:03
 0:03:20
0:03:20
 0:06:15
0:06:15
 0:13:29
0:13:29
 0:04:23
0:04:23
 0:04:37
0:04:37
 0:04:50
0:04:50
 0:03:20
0:03:20