filmov
tv
Latent heat of Fusion

Показать описание
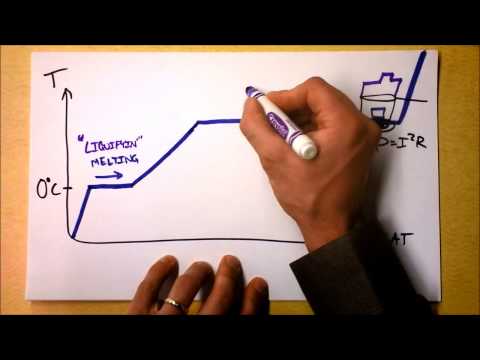
The latent heat of fusion is the amount of energy needed to change a substance from the solid phase to the liquid phase without changing its temperature. This energy, in the form of heat, is absorbed during the transition to break the bonds in the solid structure, hence no temperature change is observed. It is specific to the substance and measured in Joules per gram (J/g) or calories per gram (cal/g). For example, the latent heat of fusion for water is approximately 334 J/g.
Specific Latent Heat | Matter | Physics | FuseSchool
GCSE Physics - Specific Latent Heat #29
Latent Heat of Fusion and Vaporization | Doc Physics
Latent heat |⚡3d animation | Class 9, Chemistry |
Latent Heat Of Fusion
Class 9 - Physics - Chapter 8 - Lecture 5 - 8.5 Latent Heat of Fusion - Allied Schools
Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams
Latent heat of fusion of ice
Latent Heat of Fusion | Chemistry
Latent Heat | Chemistry
Specific heat and latent leat of fusion and vaporization | Chemistry | Khan Academy
Latent heat of Fusion
What is the latent heat of fusion Explained in 60 Seconds 🧪#vedantuclass9 #nikitamam #khusboomam
Specific Latent Heat of Fusion Definition - A Level Physics
HVAC 167 ICE latent heat of fusion
Thermodynamics: Calculating Latent and Specific Heat, Example Problem
Latent heat of fusion class 9 CBSE by Win point education
N5 Physics. Specific Latent Heat of Fusion of ice
Why is Latent heat of vaporization always larger than Latent heat of fusion?
Specific Heat and Latent Heat of fusion and vaporization
Cambridge IGCSE Physics | 2.17 Specific Latent Heat of Fusion | GCSE O Level | My Second Teacher
Measuring the Latent Heat of Fusion/Melting for Ice
Heat of Fusion and Heat of Vaporization
What is Latent heat?
Комментарии
 0:03:55
0:03:55
 0:06:26
0:06:26
 0:08:57
0:08:57
 0:03:39
0:03:39
 0:01:11
0:01:11
 0:18:04
0:18:04
 0:04:51
0:04:51
 0:04:51
0:04:51
 0:01:50
0:01:50
 0:07:15
0:07:15
 0:14:57
0:14:57
 0:00:55
0:00:55
 0:00:24
0:00:24
 0:00:13
0:00:13
 0:07:55
0:07:55
 0:06:46
0:06:46
 0:04:34
0:04:34
 0:06:32
0:06:32
 0:05:31
0:05:31
 0:14:57
0:14:57
 0:03:06
0:03:06
 0:30:15
0:30:15
 0:06:29
0:06:29
 0:00:51
0:00:51