filmov
tv
Writing Equilibrium Expressions: Kp and Kc

Показать описание
General Chemistry Problem [13-03-00]. Writing Equilibrium Expressions, Kp and Kc
#Equilibrium #Kp #Kc
------------------------------
Question:
(a) Write down the equilibrium expression for the following reaction:
2 N2O5(g) = 4 NO2(g) + O2(g)
(b) If the equilibrium constant Kp for this reaction is 0.00352 at 330 K, what is the value of the equilibrium constant Kc at this temperature?
(c) What is the equilibrium constant Kc for the reverse reaction?
------------------------------
So here is the solution.
Let's start with Part A. It tells us that the reaction is two N2O5 gas going to four NO2 gas plus O2 gas. In order to write down the equilibrium expression you must have the balanced equation, and this equation is already balanced, but you should always check.
The equilibrium expression for a reaction as usually written in terms of the partial pressure if the species is a gas. On the other hand if the species is in solution, you would use the concentration. All three species in this reaction are gases, so we are going to use their partial pressures to write down the equilibrium expression.
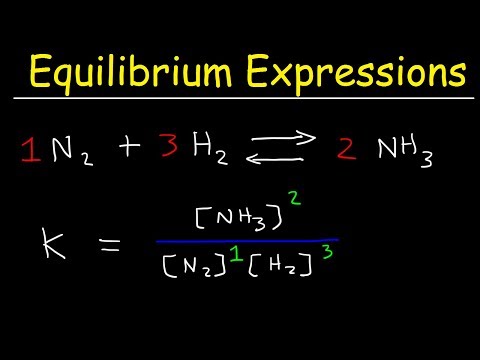
You always put the products on top and the reactants in the bottom. So here we have NO2, and we are using the partial pressure of that. And we have O2, and we're using the partial pressure of that. We always take the stoichiometric coefficients from the reaction and put them into the exponents of each of the partial pressure of the species. So you see here is 4 so we put a 4 there. Here is an implied 1, so we can put a 1 there. And now we go to the bottom. And for the reactant we have just one species, and that is N2O5, and so with the partial pressure of that, but again we have a stoichiometric coefficient of 2, so we remember to put that in the exponent. So the correct expression for Kp for this reaction is partial pressure of NO2 to the fourth power, multiplied by partial pressure of O2 ,divided by partial pressure of N2O5 to the second power. Again we use partial pressures typically if they are gases.
Part B. So in B we are given the value for Kp and we want to calculate the value for Kc. So the value for Kp we are given is equal to 0.00352. So let me just write that down -- 0.00352. And so we want to convert that to Kc. see, the difference between Kp and Kc is Kc is written in terms of concentrations. So let me write down what Kc would be before we actually calculate it. Kc is the same thing as Kp except everything is concentrations, so instead of having the partial pressure of NO2, we would have the concentration of NO2 to the fourth power. Concentration of O2 to the first power. Concentration of N2O5 to the second power. So that is the expression for Kc.
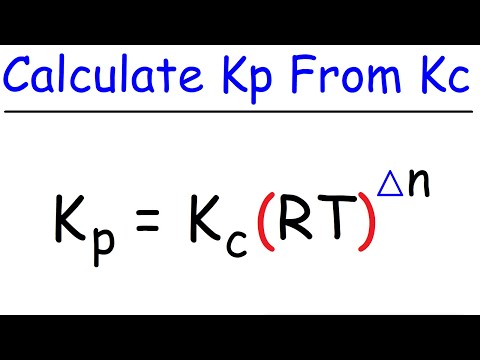
Now Kc and Kp have different values because one is written in partial pressures and the other one is written in concentrations. But they are related to each other because we know according to the ideal gas law, PV is equal to nRT, and the concentration of a gas is equal to the number of moles per volume. So you can rearrange the ideal gas law and you can see that the concentration is just equal to the partial pressure of the gas, divided by RT. So instead of the concentrations of each of these three species, we can actually write down the concentration using this expression.
So let me do that. I'm going to change to a different colo. So the NO2 concentration is the partial pressure of NO2 divided by RT, and that we raise to the power 4. The O2 concentration is the partial pressure of O2 divided by RT, and that we raised to the first power. And finally N2O5, the concentration of that is the partial pressure N2O5 divided by RT, raised to the second power.
Now I see that in this expression for Kc, I have a bunch of partial pressures, but I also have a bunch of factors of 1/RT. So I'm going to divide up the expression. Group the partial pressures together. So I have partial pressure of NO2 to the fourth power multiplied by the partial pressure of O2 to the first power, divided by the partial pressure of N2O5 to the second power. And now what's left are the rest of the factors of 1/RT. So I have 1/RT to the fourth power, 1/RT to the first power, 1/RT to the second power. So these are the factors that are actually left. From this 1/RT here to the fourth power you get that. 1/RT to the first power, you get that. 1/RT to the second power, you get that.
So these are what are left -- the factors of RT that are left. Now you notice that the first factor in this expression, which is this, is exactly just Kp as we have in part A. So whichever this is, the value of that is just Kp. And we know what that is, because the problem tells us that this is equal to 0.00352. So if we want to calculate the value for Kc, all we needed to do is to actually figure out how many factors of 1/RT we have in the second factor, and we just multiply that by Kp.
.....
#Equilibrium #Kp #Kc
------------------------------
Question:
(a) Write down the equilibrium expression for the following reaction:
2 N2O5(g) = 4 NO2(g) + O2(g)
(b) If the equilibrium constant Kp for this reaction is 0.00352 at 330 K, what is the value of the equilibrium constant Kc at this temperature?
(c) What is the equilibrium constant Kc for the reverse reaction?
------------------------------
So here is the solution.
Let's start with Part A. It tells us that the reaction is two N2O5 gas going to four NO2 gas plus O2 gas. In order to write down the equilibrium expression you must have the balanced equation, and this equation is already balanced, but you should always check.
The equilibrium expression for a reaction as usually written in terms of the partial pressure if the species is a gas. On the other hand if the species is in solution, you would use the concentration. All three species in this reaction are gases, so we are going to use their partial pressures to write down the equilibrium expression.
You always put the products on top and the reactants in the bottom. So here we have NO2, and we are using the partial pressure of that. And we have O2, and we're using the partial pressure of that. We always take the stoichiometric coefficients from the reaction and put them into the exponents of each of the partial pressure of the species. So you see here is 4 so we put a 4 there. Here is an implied 1, so we can put a 1 there. And now we go to the bottom. And for the reactant we have just one species, and that is N2O5, and so with the partial pressure of that, but again we have a stoichiometric coefficient of 2, so we remember to put that in the exponent. So the correct expression for Kp for this reaction is partial pressure of NO2 to the fourth power, multiplied by partial pressure of O2 ,divided by partial pressure of N2O5 to the second power. Again we use partial pressures typically if they are gases.
Part B. So in B we are given the value for Kp and we want to calculate the value for Kc. So the value for Kp we are given is equal to 0.00352. So let me just write that down -- 0.00352. And so we want to convert that to Kc. see, the difference between Kp and Kc is Kc is written in terms of concentrations. So let me write down what Kc would be before we actually calculate it. Kc is the same thing as Kp except everything is concentrations, so instead of having the partial pressure of NO2, we would have the concentration of NO2 to the fourth power. Concentration of O2 to the first power. Concentration of N2O5 to the second power. So that is the expression for Kc.
Now Kc and Kp have different values because one is written in partial pressures and the other one is written in concentrations. But they are related to each other because we know according to the ideal gas law, PV is equal to nRT, and the concentration of a gas is equal to the number of moles per volume. So you can rearrange the ideal gas law and you can see that the concentration is just equal to the partial pressure of the gas, divided by RT. So instead of the concentrations of each of these three species, we can actually write down the concentration using this expression.
So let me do that. I'm going to change to a different colo. So the NO2 concentration is the partial pressure of NO2 divided by RT, and that we raise to the power 4. The O2 concentration is the partial pressure of O2 divided by RT, and that we raised to the first power. And finally N2O5, the concentration of that is the partial pressure N2O5 divided by RT, raised to the second power.
Now I see that in this expression for Kc, I have a bunch of partial pressures, but I also have a bunch of factors of 1/RT. So I'm going to divide up the expression. Group the partial pressures together. So I have partial pressure of NO2 to the fourth power multiplied by the partial pressure of O2 to the first power, divided by the partial pressure of N2O5 to the second power. And now what's left are the rest of the factors of 1/RT. So I have 1/RT to the fourth power, 1/RT to the first power, 1/RT to the second power. So these are the factors that are actually left. From this 1/RT here to the fourth power you get that. 1/RT to the first power, you get that. 1/RT to the second power, you get that.
So these are what are left -- the factors of RT that are left. Now you notice that the first factor in this expression, which is this, is exactly just Kp as we have in part A. So whichever this is, the value of that is just Kp. And we know what that is, because the problem tells us that this is equal to 0.00352. So if we want to calculate the value for Kc, all we needed to do is to actually figure out how many factors of 1/RT we have in the second factor, and we just multiply that by Kp.
.....
 0:05:24
0:05:24
 0:04:47
0:04:47
 0:16:58
0:16:58
 0:04:32
0:04:32
 0:07:31
0:07:31
 0:10:20
0:10:20
 0:53:22
0:53:22
 0:03:04
0:03:04
 0:17:53
0:17:53
 0:04:46
0:04:46
 0:10:51
0:10:51
 0:06:48
0:06:48
 0:07:03
0:07:03
 0:05:13
0:05:13
 0:07:37
0:07:37
 0:07:57
0:07:57
 0:06:27
0:06:27
 0:04:43
0:04:43
 0:01:31
0:01:31
 0:04:46
0:04:46
 0:01:29
0:01:29
 0:07:15
0:07:15
 0:05:37
0:05:37
 0:28:41
0:28:41