filmov
tv
Calculating Empirical Formula

Показать описание
Empirical Formula are a popular question on the exams. This video shows a simple method that will allow you to throw out an empirical formula in minutes! Not only that there is a way in which you can turn empirical formula into molecular formula.
Empirical Formula & Molecular Formula Determination From Percent Composition
Finding and Calculating an Empirical Formula of a Compound | How to Pass Chemistry
How to calculate Empirical Formula? 3 Easy Steps
Empirical Formulae From Percentage Composition | Chemical Calculations | Chemistry | FuseSchool
Empirical Formula and Molecular Formula Introduction
Calculating Empirical Formula
Calculating Molecular Formula from Empirical Formula
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
CHEMISTRY 101: Finding Empirical Formula Using Combustion Analysis for a Compound with C, H, O
Writing Empirical Formula Practice Problems
Empirical Formula: How to calculate | Stoichiometry | Chemistry
Empirical Formula Grade 11 Exam
Practice Problem: Empirical and Molecular Formulas
Calculating Empirical Formulas with Percent Composition
How to Calculate EMPIRICAL FORMULA Using 5 Simple Steps
How to Find the Empirical Formula of C6H12O6
3.6 Determination of Empirical Formula
Empirical and molecular formula grade 11
Calculating the Empirical formula | Stoichiometry | Grade 11
Determining the Empirical Formula from a Percent
Calculating Molecular Formula from Empirical Formula
Empirical formula calculation
Determining the empirical formula using combustion Analysis
Empirical Formula and Molecular Formula | Basic Concept | Numerical Problems
Комментарии
 0:11:00
0:11:00
 0:02:52
0:02:52
 0:05:01
0:05:01
 0:04:33
0:04:33
 0:08:31
0:08:31
 0:06:37
0:06:37
 0:09:09
0:09:09
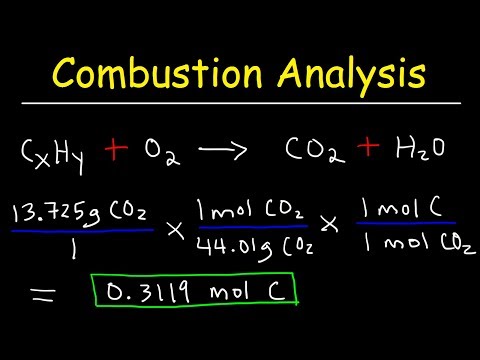 0:16:49
0:16:49
 0:04:12
0:04:12
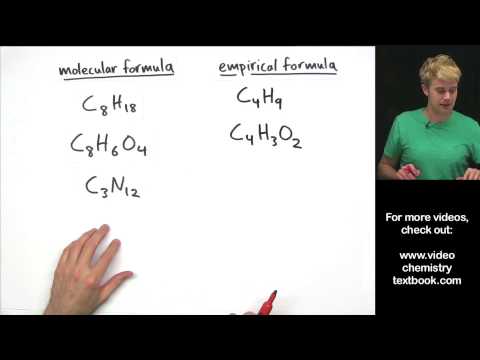 0:06:09
0:06:09
 0:08:50
0:08:50
 0:05:08
0:05:08
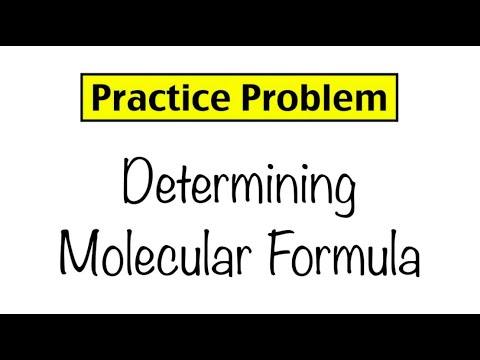 0:05:53
0:05:53
 0:06:51
0:06:51
 0:04:17
0:04:17
 0:00:52
0:00:52
 0:11:06
0:11:06
 0:13:01
0:13:01
 0:21:20
0:21:20
 0:03:40
0:03:40
 0:06:06
0:06:06
 0:11:04
0:11:04
 0:09:36
0:09:36
 0:13:30
0:13:30