filmov
tv
Medical Devices classification as per FDA | Medical Device Regulations | #MedicalDevices #FDA
Показать описание
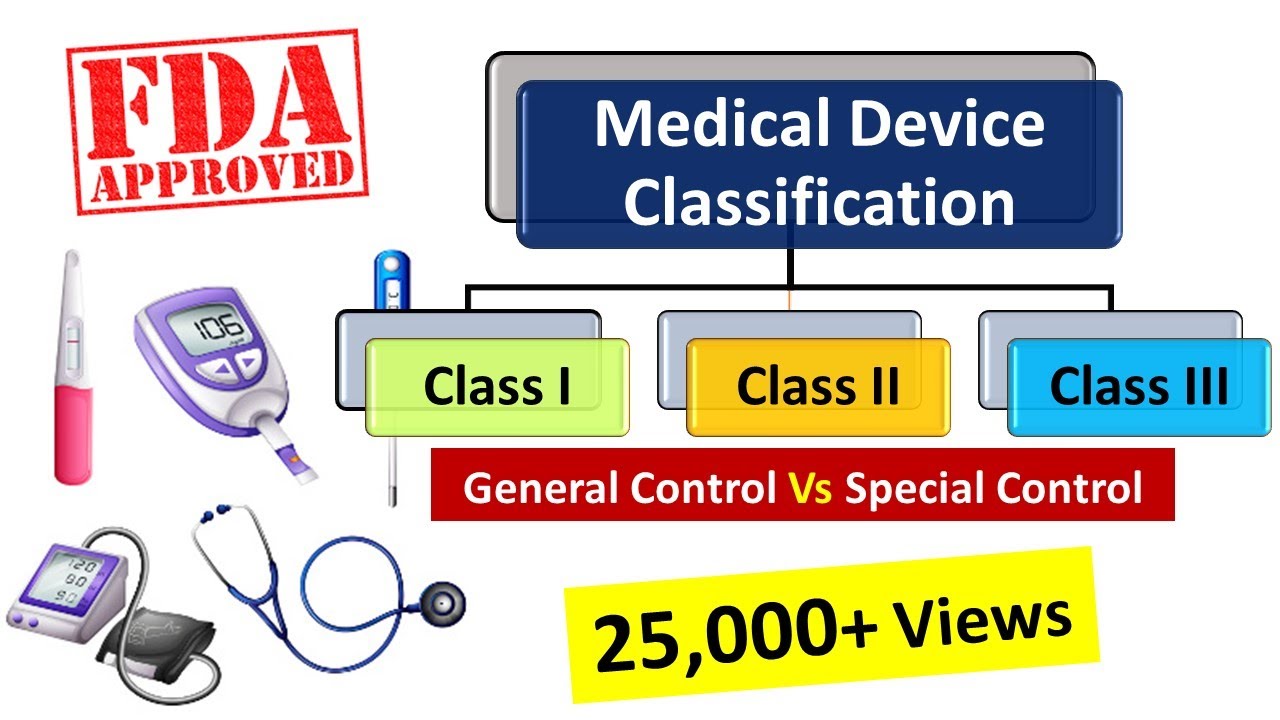
Devices are classified into one of three regulatory classes: class I, class II, or class III. Watch the video for more details and share it with all your friends on social media.
⏱️ 𝑻𝒊𝒎𝒆 𝑺𝒕𝒂𝒎𝒑 ⏱️
-------------------------------
0:00 - Introduction
📢📢 𝑺𝒐𝒄𝒊𝒂𝒍 𝑴𝒆𝒅𝒊𝒂 𝑳𝒊𝒏𝒌:
--------------------------------------------------
𝐉𝐨𝐢𝐧 my 𝐓𝐞𝐥𝐞𝐠𝐫𝐚𝐦 𝐂𝐡𝐚𝐧𝐧𝐞𝐥𝐬 for regular updates:
--------------------------------------------------------------------------------
𝐉𝐨𝐢𝐧 my 𝐖𝐡𝐚𝐭𝐬𝐀𝐩𝐩 𝐂𝐡𝐚𝐧𝐧𝐞𝐥𝐬 for regular updates:
--------------------------------------------------------------------------------
✍️ 𝓓𝓲𝓼𝓬𝓵𝓪𝓲𝓶𝓮𝓻:
Copyright Disclaimer under section 107 of the Copyright Act of 1976, allowance is made for “fair use” for purposes such as criticism, comment, news reporting, teaching, scholarship, education and research. Fair use is a use permitted by copyright statute that might otherwise be infringing. The information contained in this video is just for educational and informational purposes only and does not have any intention to mislead or violate Google and YouTube community guidelines or policy. I respect and follow all terms & conditions of Google & YouTube.
𝐂𝐨𝐦𝐦𝐨𝐧 𝐊𝐞𝐲 𝐬𝐞𝐚𝐫𝐜𝐡 𝐫𝐞𝐥𝐚𝐭𝐞𝐝 𝐭𝐨 𝐭𝐡𝐢𝐬 𝐭𝐨𝐩𝐢𝐜 :
----------------------------------------------------------------------
⏱️ 𝑻𝒊𝒎𝒆 𝑺𝒕𝒂𝒎𝒑 ⏱️
-------------------------------
0:00 - Introduction
📢📢 𝑺𝒐𝒄𝒊𝒂𝒍 𝑴𝒆𝒅𝒊𝒂 𝑳𝒊𝒏𝒌:
--------------------------------------------------
𝐉𝐨𝐢𝐧 my 𝐓𝐞𝐥𝐞𝐠𝐫𝐚𝐦 𝐂𝐡𝐚𝐧𝐧𝐞𝐥𝐬 for regular updates:
--------------------------------------------------------------------------------
𝐉𝐨𝐢𝐧 my 𝐖𝐡𝐚𝐭𝐬𝐀𝐩𝐩 𝐂𝐡𝐚𝐧𝐧𝐞𝐥𝐬 for regular updates:
--------------------------------------------------------------------------------
✍️ 𝓓𝓲𝓼𝓬𝓵𝓪𝓲𝓶𝓮𝓻:
Copyright Disclaimer under section 107 of the Copyright Act of 1976, allowance is made for “fair use” for purposes such as criticism, comment, news reporting, teaching, scholarship, education and research. Fair use is a use permitted by copyright statute that might otherwise be infringing. The information contained in this video is just for educational and informational purposes only and does not have any intention to mislead or violate Google and YouTube community guidelines or policy. I respect and follow all terms & conditions of Google & YouTube.
𝐂𝐨𝐦𝐦𝐨𝐧 𝐊𝐞𝐲 𝐬𝐞𝐚𝐫𝐜𝐡 𝐫𝐞𝐥𝐚𝐭𝐞𝐝 𝐭𝐨 𝐭𝐡𝐢𝐬 𝐭𝐨𝐩𝐢𝐜 :
----------------------------------------------------------------------
Комментарии
 0:10:59
0:10:59
 0:16:18
0:16:18
 0:03:12
0:03:12
 0:12:24
0:12:24
 0:08:23
0:08:23
 0:03:28
0:03:28
 0:14:55
0:14:55
 0:11:24
0:11:24
 0:03:02
0:03:02
 0:09:28
0:09:28
 0:03:57
0:03:57
 0:05:55
0:05:55
 0:05:21
0:05:21
 0:05:59
0:05:59
 0:00:33
0:00:33
 0:31:51
0:31:51
 0:03:05
0:03:05
 0:04:48
0:04:48
 1:01:14
1:01:14
 0:01:23
0:01:23
 0:04:38
0:04:38
 0:03:37
0:03:37
 0:28:40
0:28:40
 0:13:34
0:13:34