filmov
tv
United States Medical Device Registration Chapter 2 - Classification

Показать описание
The US market represents more than 40% of the global market for medical devices. Yet for many manufacturers, the process of obtaining clearance from the US FDA can be daunting. In this seven part slidecast we will demystify the FDA device clearance process, breaking it down into understandable steps and explaining each one. Once you begin the series you may skip to the next section at any time using the Playlist in the player window. Information current as of June 2014.
In this SlideCast:
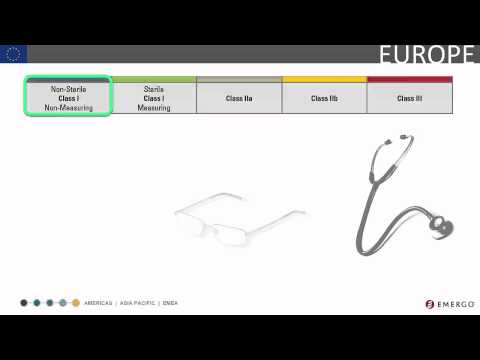
The steps involved in classifying your medical device [3:56]
In this SlideCast:
The steps involved in classifying your medical device [3:56]
 0:03:59
0:03:59
 0:05:13
0:05:13
 0:03:57
0:03:57
 0:03:25
0:03:25
 0:04:46
0:04:46
 0:04:07
0:04:07
 0:02:08
0:02:08
 0:03:09
0:03:09
 1:06:09
1:06:09
 0:02:40
0:02:40
 0:03:21
0:03:21
 0:02:24
0:02:24
 0:02:29
0:02:29
 0:06:45
0:06:45
 0:09:28
0:09:28
 0:03:26
0:03:26
 0:04:04
0:04:04
 0:03:37
0:03:37
 0:21:10
0:21:10
 0:04:36
0:04:36
 0:02:19
0:02:19
 0:03:01
0:03:01
 0:16:18
0:16:18
 0:23:24
0:23:24