filmov
tv
IGCSE Chemistry 0620 | Chapter 6 - Chemical Energetics
Показать описание
In todays video we look at Chemical energetics, the topics taught are:
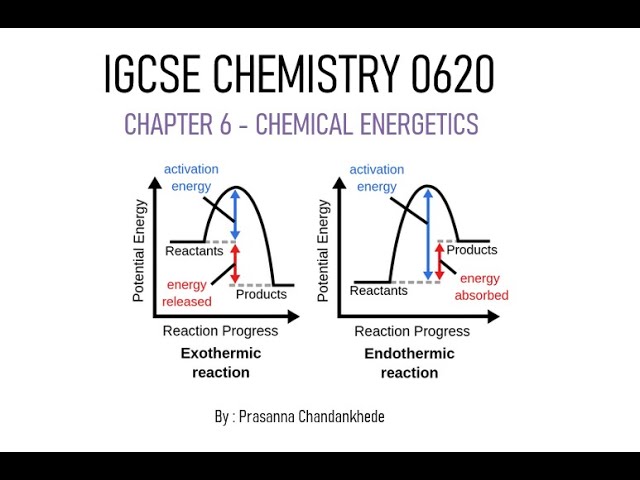
Energetics of a reaction - Endothermic reactions and Exothermic reactions and energy level diagrams
Endothermic and exothermic reactions are two fundamental types of chemical reactions that are categorized based on the energy changes that occur during the reaction. These energy changes are typically associated with the heat flow into or out of the system.
Endothermic Reactions:
In an endothermic reaction, the system absorbs heat or energy from its surroundings.
This means that the products of the reaction have more energy than the reactants, and as a result, the surroundings experience a decrease in temperature.
Endothermic reactions often feel cold to the touch because they draw heat away from their surroundings.
Examples of endothermic reactions include:
Photosynthesis: The process by which plants convert carbon dioxide and water into glucose and oxygen, using energy from sunlight.
Evaporation of Water: When liquid water turns into water vapor, it absorbs heat from the surroundings, causing cooling.
Melting of Ice: The solid ice absorbs heat to become liquid water.
Exothermic Reactions:
In an exothermic reaction, the system releases heat or energy into its surroundings.
This means that the products of the reaction have less energy than the reactants, and the surroundings experience an increase in temperature.
Exothermic reactions often feel warm or hot to the touch because they transfer heat to their surroundings.
Examples of exothermic reactions include:
Combustion of Fuels: Burning gasoline in a car engine or wood in a fireplace releases heat and energy into the surroundings.
Chemical Explosions: Explosive reactions, like the detonation of dynamite, release a significant amount of heat and energy.
Neutralization Reactions: The reaction between an acid and a base to form water and a salt typically releases heat.
It's important to note that the terms "endothermic" and "exothermic" refer to the net energy change in the reaction. In an endothermic reaction, energy is absorbed, while in an exothermic reaction, energy is released. These concepts are crucial in various fields, including chemistry, thermodynamics, and physics, as they help in understanding the behavior of substances and reactions in different environments.
Energetics of a reaction - Endothermic reactions and Exothermic reactions and energy level diagrams
Endothermic and exothermic reactions are two fundamental types of chemical reactions that are categorized based on the energy changes that occur during the reaction. These energy changes are typically associated with the heat flow into or out of the system.
Endothermic Reactions:
In an endothermic reaction, the system absorbs heat or energy from its surroundings.
This means that the products of the reaction have more energy than the reactants, and as a result, the surroundings experience a decrease in temperature.
Endothermic reactions often feel cold to the touch because they draw heat away from their surroundings.
Examples of endothermic reactions include:
Photosynthesis: The process by which plants convert carbon dioxide and water into glucose and oxygen, using energy from sunlight.
Evaporation of Water: When liquid water turns into water vapor, it absorbs heat from the surroundings, causing cooling.
Melting of Ice: The solid ice absorbs heat to become liquid water.
Exothermic Reactions:
In an exothermic reaction, the system releases heat or energy into its surroundings.
This means that the products of the reaction have less energy than the reactants, and the surroundings experience an increase in temperature.
Exothermic reactions often feel warm or hot to the touch because they transfer heat to their surroundings.
Examples of exothermic reactions include:
Combustion of Fuels: Burning gasoline in a car engine or wood in a fireplace releases heat and energy into the surroundings.
Chemical Explosions: Explosive reactions, like the detonation of dynamite, release a significant amount of heat and energy.
Neutralization Reactions: The reaction between an acid and a base to form water and a salt typically releases heat.
It's important to note that the terms "endothermic" and "exothermic" refer to the net energy change in the reaction. In an endothermic reaction, energy is absorbed, while in an exothermic reaction, energy is released. These concepts are crucial in various fields, including chemistry, thermodynamics, and physics, as they help in understanding the behavior of substances and reactions in different environments.
 0:34:57
0:34:57
 0:14:51
0:14:51
 0:17:56
0:17:56
 0:37:58
0:37:58
 0:17:29
0:17:29
 0:16:06
0:16:06
 0:18:26
0:18:26
 0:12:45
0:12:45
 0:21:29
0:21:29
 0:17:24
0:17:24
 0:12:50
0:12:50
 0:28:12
0:28:12
 0:14:03
0:14:03
 0:03:55
0:03:55
 0:15:30
0:15:30
 0:49:23
0:49:23
 0:08:12
0:08:12
 0:07:51
0:07:51
 0:08:58
0:08:58
 0:27:37
0:27:37
 0:09:02
0:09:02
 0:06:56
0:06:56
 0:10:01
0:10:01
 0:00:31
0:00:31