filmov
tv
Solubility Rules and Predicting Reactions

Показать описание
Solubility Rules and Predicting Reactions. Mr. Causey shows you step by step how to find the products of a double replacement reaction and how to determine if a solid will precipitate out of the solution.
SUBSCRIBE for more chemistry videos:
CONTACT ME:
FOLLOW ME:
RELATED VIDEOS:
Balancing Equations:
Aqueous Solutions:
Solubility Rules:
RESOURCES
Polyatomic Ion Cheat Sheet:
Periodic Table:
ABOUT MR. CAUSEY'S VIDEO ACADEMY
Mr. Causey's Video Academy is an educational video series of short video lessons for chemistry, algebra, and physics. You can get lessons on a variety of topics or homework helpers that show you how to solve certain problems. There are over 120 videos from which to choose.
#mrcausey #yourchemcoach #predictingreactions
SUBSCRIBE for more chemistry videos:
CONTACT ME:
FOLLOW ME:
RELATED VIDEOS:
Balancing Equations:
Aqueous Solutions:
Solubility Rules:
RESOURCES
Polyatomic Ion Cheat Sheet:
Periodic Table:
ABOUT MR. CAUSEY'S VIDEO ACADEMY
Mr. Causey's Video Academy is an educational video series of short video lessons for chemistry, algebra, and physics. You can get lessons on a variety of topics or homework helpers that show you how to solve certain problems. There are over 120 videos from which to choose.
#mrcausey #yourchemcoach #predictingreactions
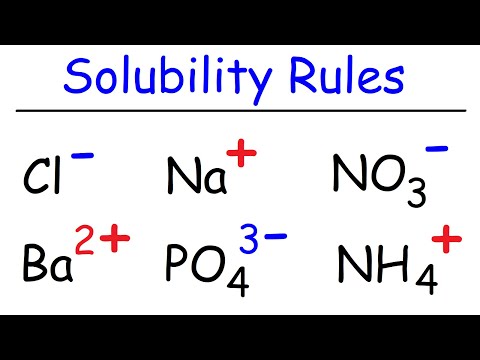
Solubility Rules
Solubility Rules and Predicting Reactions
Precipitation Reactions - Using the Solubility Rules
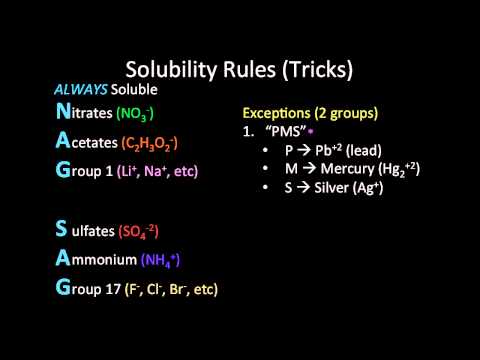
Solubility Rules (Mnemonic Tricks)
Solubility Rules and How to Use a Solubility Table
Solubility Rules | Acids, Bases & Alkali's | Chemistry | FuseSchool
Precipitation reactions, predicting products and solubility rules explained
Chemistry - Will The Reaction Occur?
Solubility Rules: Explanation & Practice
Precipitation Reactions: Crash Course Chemistry #9
94: Precipitation reactions & solubility rules
Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems
Precipitation reactions, simple trick to predict products and solubility rules
Solubility Rules, Predicting Precipitates and Net Ionic Equations
Predicting chemical reactions using solubility rules DAY 2
Predicting Products of Double Replacement Reactions
Precipitation Reactions and Solubility Rules
Chemical Reactions Using Solubility Rules to Predict Products in Reactions
Predicting Double Replacement Reactions Part 1 (Solubility Rules)
Predicting Chemical Reactions Using Solubility Rules
Ionic Reactions, Precipitation Reactions and Solubility Rules
Soluble and Insoluble Salts // Easy Way to Remember!
Dr. Udell Honors Chemistry 4 2 precipitation reactions solubility rules and predicting products 2013
Chemistry 30 Solubility Rules and predicting precipitation
Комментарии
 0:06:19
0:06:19
 0:05:39
0:05:39
 0:10:37
0:10:37
 0:02:41
0:02:41
 0:07:35
0:07:35
 0:04:45
0:04:45
 0:18:21
0:18:21
 0:12:44
0:12:44
 0:09:31
0:09:31
 0:11:31
0:11:31
 0:13:39
0:13:39
 0:18:42
0:18:42
 0:18:21
0:18:21
 0:22:03
0:22:03
 0:08:51
0:08:51
 0:02:49
0:02:49
 0:23:18
0:23:18
 0:15:35
0:15:35
 0:04:13
0:04:13
 0:17:28
0:17:28
 0:18:20
0:18:20
 0:04:50
0:04:50
 0:14:31
0:14:31
 0:20:15
0:20:15