filmov
tv
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes - Chemistry Examples
Показать описание
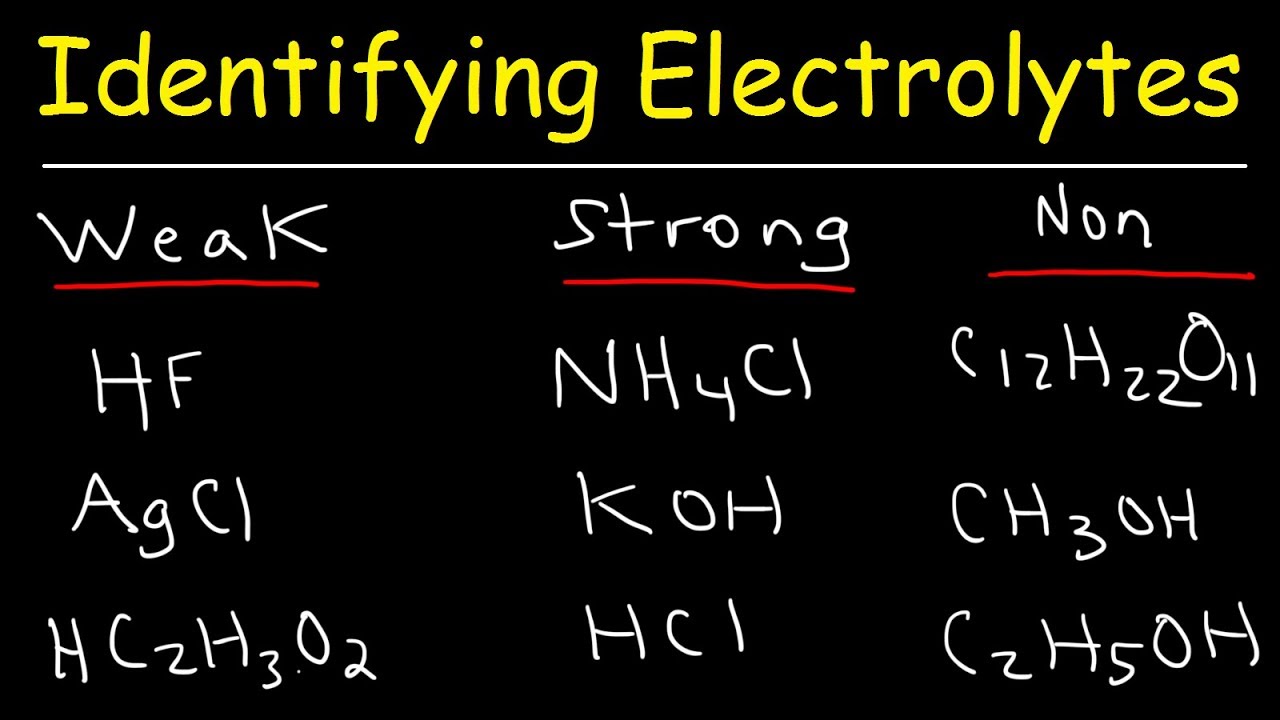
This chemistry video tutorial explains how to identify weak electrolytes, strong electrolytes, and nonelectrolytes. Strong electrolytes including strong acids and bases as well as soluble ionic compounds that ionize completely in solution. Weak electrolytes include weak acids and bases and insoluble ionic compounds that ionizes partially in aqueous solution. Nonelectrolytes may be soluble in water but they do not ionize. Sugar and alcohol mixed with water are common nonelectrolytes that do not conduct electricity.
Stoichiometry Practice Test:
Solute, Solvent, & Solution:
Strong & Weak Electrolytes:
Molarity Practice Problems:
Ion Concentration In Solutions:
Dilution Problems:
___________________________________
Types of Chemical Reactions:
Solubility Rules:
Predicting The Products of Reactions:
Activity Series of Metals:
Will This Reaction Occur?
Predicting Products of SR Reactions:
___________________________________
Double Replacement Reactions:
Net Ionic Equations:
Writing Chemical Equations From Words:
Solution Stoichiometry:
Molarity & Dilution Problems:
Acid Base Neutralization Reactions:
____________________________________
Acid Base Titration Problems:
Mixture Problems:
Calculating Oxidation Numbers:
Oxidation and Reduction Reactions:
Balancing Redox Reactions:
Ideal Gas Law Problems:
___________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Stoichiometry Practice Test:
Solute, Solvent, & Solution:
Strong & Weak Electrolytes:
Molarity Practice Problems:
Ion Concentration In Solutions:
Dilution Problems:
___________________________________
Types of Chemical Reactions:
Solubility Rules:
Predicting The Products of Reactions:
Activity Series of Metals:
Will This Reaction Occur?
Predicting Products of SR Reactions:
___________________________________
Double Replacement Reactions:
Net Ionic Equations:
Writing Chemical Equations From Words:
Solution Stoichiometry:
Molarity & Dilution Problems:
Acid Base Neutralization Reactions:
____________________________________
Acid Base Titration Problems:
Mixture Problems:
Calculating Oxidation Numbers:
Oxidation and Reduction Reactions:
Balancing Redox Reactions:
Ideal Gas Law Problems:
___________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Комментарии
 0:10:13
0:10:13
 0:05:44
0:05:44
 0:10:38
0:10:38
 0:16:37
0:16:37
 0:07:48
0:07:48
 0:05:28
0:05:28
 0:22:19
0:22:19
 0:09:20
0:09:20
 0:09:55
0:09:55
 0:04:49
0:04:49
 0:02:10
0:02:10
 0:01:25
0:01:25
 0:07:18
0:07:18
 0:05:03
0:05:03
 0:07:50
0:07:50
 0:04:59
0:04:59
 0:00:05
0:00:05
 0:01:27
0:01:27
 0:02:34
0:02:34
 0:10:31
0:10:31
 0:11:33
0:11:33
 0:14:15
0:14:15
 0:05:52
0:05:52
 0:03:32
0:03:32