filmov
tv
Calculate pH from H+ concentration 008

Показать описание
Calculate the pH of a solution in which the hydrogen ion concentration is 3.6 x 10-4 M.
Calculating pH From Hydronium Ion Concentration
How to Calculate Hydrogen Ion Concentration from pH
How to Calculate hydrogen ion concentration [H+] FROM pH | pH CALCULATION | BIOCHEMISTRY
Calculate pH from H+ concentration 008
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems
How to find pH, pOH, H3O+, and OH- STEP BY STEP
A level Chemistry Converting pH into hydrogen ion concentration
Find the Hydronium Ion Concentration given the pH
IONIC EQUILIBRIUM REVISION
Calculating pH from hydrogen or hydroxide ion concentration
Determine PH of a solution given the hydrogen concentration
Calculating Hydrogen Ion Concentration
Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE
How to calculate concentration from pH and pOH
Calculate pH given OH concentration
How to find concentration of H+ given pH
Given pH & pOH, Solve for [H+] & [OH-] Practice Problems
Calculating pH from hydrogen ion (hydronium ion) concentration
Calculate the pH of Acids and Bases Given the Concentration of a Solution
How To Calculate The pH of a Solution Without a Calculator - Acids and Bases
More pH problems - Calculate hydrogen ion concentration from pH 001
Given [H+] or [OH-], Calculate pH & pOH
Hydrogen Ion and Hydroxide Ion Concentrations (Example)
Logarithmic Application - pH and Hydrogen Ion Concentration
Комментарии
 0:02:29
0:02:29
 0:01:57
0:01:57
 0:03:39
0:03:39
 0:01:54
0:01:54
 0:13:50
0:13:50
 0:04:05
0:04:05
 0:03:13
0:03:13
 0:02:00
0:02:00
 1:34:02
1:34:02
 0:13:03
0:13:03
 0:04:21
0:04:21
 0:10:14
0:10:14
 0:09:14
0:09:14
 0:03:14
0:03:14
 0:01:24
0:01:24
 0:03:48
0:03:48
 0:08:38
0:08:38
 0:08:17
0:08:17
 0:28:17
0:28:17
 0:21:09
0:21:09
 0:03:09
0:03:09
![Given [H+] or](https://i.ytimg.com/vi/ghIYaqo0Ycc/hqdefault.jpg) 0:09:19
0:09:19
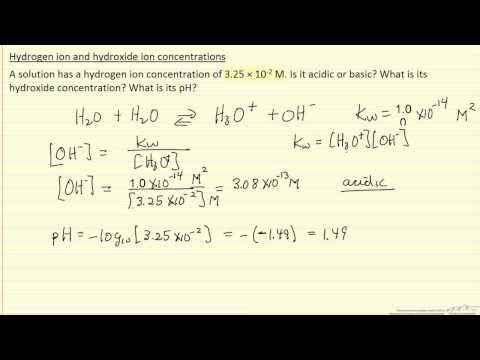 0:02:09
0:02:09
 0:03:34
0:03:34