filmov
tv
Quick overview of fatty acid metabolism

Показать описание
Key points about lipid metabolism:
* All tissues need fatty acids for various purposes: use as fuel, incorporation into membranes, production of steroids, etc. But “only” liver cells (hepatocytes) & fat cells (adipocytes) can make them
* The liver & adipocytes make fatty acids, store them (as triacylglycerides (TAGs)), & ship them out to other tissues in need.
* These fatty acids have to be “mobilized” from triacylglycerides (TAG) stores, as described below
* Fatty acids are made from acetyl-CoA (2 carbon (2C)) and broken down to acetyl-CoA (and one propionyl-CoA (3C) per odd-chain fatty acid)
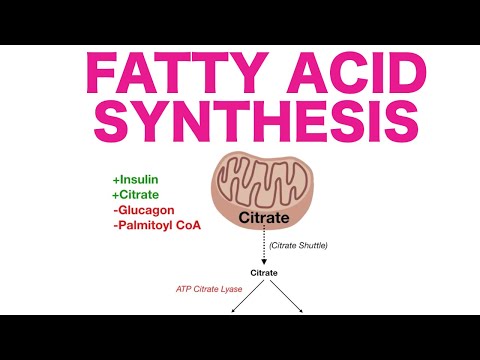
* Fatty acid synthesis occurs (mostly*) in the cytoplasm of liver & fat cells and is a key user of NADPH (which can be made through the pentose phosphate pathway (PPP)
* *some in mitochondria
* Because acetyl-CoA can’t get through the mitochondrial membranes, citrate, not acetyl-CoA, is removed from the mitochondria to make fats - it is subsequently broken down back to acetyl-CoA by ATP-citrate lyase
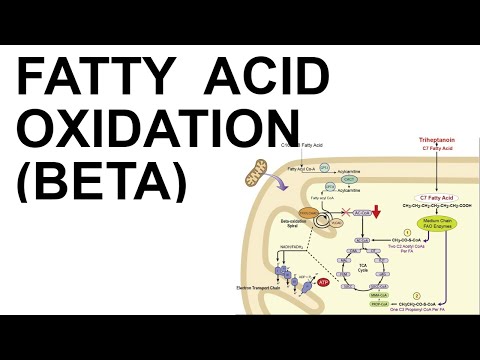
* Fatty acid breakdown occurs in most tissues & takes place (mostly*) in the mitochondria in a process called β-oxidation
* *very long ones (over 20C) are initially via a hydrogen-peroxide mediated process in peroxisomes
* Synthesis & breakdown are reciprocally regulated
* Key regulatory points are:
* Synthesis: acetyl-CoA carboxylase (ACC), which activates acetyl-CoA for incorporation
* Breakdown: carnitine-acyltransferase 1 (CAT-1/CPT-1), which allows fatty acids into the mitochondria for breakdown
Fat mobilization
* Cells take up fatty acids, not TAGs, for use as fuel, so fatty acids need to be cleaved off of the glycerol backbone (by lipases) for uptake & subsequent use
* Fatty acids are delivered from liver cells to tissues packaged as TAGs, bundled up with phospholipids, cholesterol, & other hydrophobic stuff in the interior of lipid-coated “bubbles” called lipoproteins
* The fatty acids are freed from TAGs for uptake by other tissues through the action of lipoprotein lipase on the surface of blood vessels
* Fatty acids from adipocytes are delivered as fatty acids, not in lipoproteins
* Since fatty acids are hydrophobic, these travel through the bloodstream by piggybacking on proteins like serum albumin that have hydrophobic binding patches
* Hormone-sensitive lipase, activated by adrenaline & glucagon (hormone signaling low blood sugar), breaks fatty acids off of TAGs inside of fat & liver cells (as opposed to lipoprotein lipase, which acts extracellularly to get fatty acids into cells)
* This helps “mobilize” fuel stores for breakdown for energy inside the cell or shipping out to other cells
Fatty acid synthesis
* Fatty acid synthesis occurs (mostly*) in the cytoplasm of liver & fat cells and is a key user of NADPH (which can be made through the pentose phosphate pathway (PPP)
* *some in mitochondria
* Because acetyl-CoA can’t get through the mitochondrial membranes, citrate, not acetyl-CoA, is removed from the mitochondria to make fats - it is subsequently broken down back to acetyl-CoA by ATP-citrate lyase
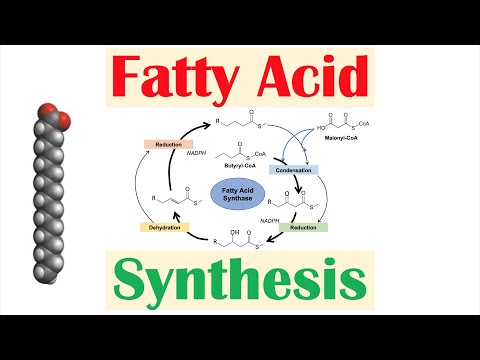
* Fatty acids are built by a multifunctional protein called fatty acid synthase (FAS), 2C at a time, from 3C intermediates (malonyl-CoA)
* See diagram for details
* Malonyl-CoA is made by carboxylation (from bicarb) of acetyl-CoA by acetyl-CoA carboxylase (ACC)
* this step activates acetyl-CoA by making a β-keto acid (energetically-favorable to subsequently decarboxylate once linked on to the chain)
* the carbon that is added from CO2 is subsequently lost and not incorporated into the fatty acid
* key site of regulation
* activated by citrate (feed-forward stimulation) & insulin (via activation of a phosphatase)
* inhibited by palmitoyl-CoA (feedback inhibition) & phosphorylation via PKA (stimulated by glucagon & epinephrine) or AMPK (stimulated by high AMP levels)
* malonyl-CoA itself (a signal of lipid synthesis) is an inhibitor of CAT-1/CPT-1, the transporter that lets fatty acids into mitochondria for breakdown
* It costs 1 ATP & 2 NADPH per 2C added
* The “default” fatty acid is a 16C saturated fatty acid, palmitate, which gets cleaved off of FAS by the thioesterase subunit of FAS
* Longer fatty acids & unsaturated fatty acids can be made via elongation & desaturation in the ER
Finished in comments
* All tissues need fatty acids for various purposes: use as fuel, incorporation into membranes, production of steroids, etc. But “only” liver cells (hepatocytes) & fat cells (adipocytes) can make them
* The liver & adipocytes make fatty acids, store them (as triacylglycerides (TAGs)), & ship them out to other tissues in need.
* These fatty acids have to be “mobilized” from triacylglycerides (TAG) stores, as described below
* Fatty acids are made from acetyl-CoA (2 carbon (2C)) and broken down to acetyl-CoA (and one propionyl-CoA (3C) per odd-chain fatty acid)
* Fatty acid synthesis occurs (mostly*) in the cytoplasm of liver & fat cells and is a key user of NADPH (which can be made through the pentose phosphate pathway (PPP)
* *some in mitochondria
* Because acetyl-CoA can’t get through the mitochondrial membranes, citrate, not acetyl-CoA, is removed from the mitochondria to make fats - it is subsequently broken down back to acetyl-CoA by ATP-citrate lyase
* Fatty acid breakdown occurs in most tissues & takes place (mostly*) in the mitochondria in a process called β-oxidation
* *very long ones (over 20C) are initially via a hydrogen-peroxide mediated process in peroxisomes
* Synthesis & breakdown are reciprocally regulated
* Key regulatory points are:
* Synthesis: acetyl-CoA carboxylase (ACC), which activates acetyl-CoA for incorporation
* Breakdown: carnitine-acyltransferase 1 (CAT-1/CPT-1), which allows fatty acids into the mitochondria for breakdown
Fat mobilization
* Cells take up fatty acids, not TAGs, for use as fuel, so fatty acids need to be cleaved off of the glycerol backbone (by lipases) for uptake & subsequent use
* Fatty acids are delivered from liver cells to tissues packaged as TAGs, bundled up with phospholipids, cholesterol, & other hydrophobic stuff in the interior of lipid-coated “bubbles” called lipoproteins
* The fatty acids are freed from TAGs for uptake by other tissues through the action of lipoprotein lipase on the surface of blood vessels
* Fatty acids from adipocytes are delivered as fatty acids, not in lipoproteins
* Since fatty acids are hydrophobic, these travel through the bloodstream by piggybacking on proteins like serum albumin that have hydrophobic binding patches
* Hormone-sensitive lipase, activated by adrenaline & glucagon (hormone signaling low blood sugar), breaks fatty acids off of TAGs inside of fat & liver cells (as opposed to lipoprotein lipase, which acts extracellularly to get fatty acids into cells)
* This helps “mobilize” fuel stores for breakdown for energy inside the cell or shipping out to other cells
Fatty acid synthesis
* Fatty acid synthesis occurs (mostly*) in the cytoplasm of liver & fat cells and is a key user of NADPH (which can be made through the pentose phosphate pathway (PPP)
* *some in mitochondria
* Because acetyl-CoA can’t get through the mitochondrial membranes, citrate, not acetyl-CoA, is removed from the mitochondria to make fats - it is subsequently broken down back to acetyl-CoA by ATP-citrate lyase
* Fatty acids are built by a multifunctional protein called fatty acid synthase (FAS), 2C at a time, from 3C intermediates (malonyl-CoA)
* See diagram for details
* Malonyl-CoA is made by carboxylation (from bicarb) of acetyl-CoA by acetyl-CoA carboxylase (ACC)
* this step activates acetyl-CoA by making a β-keto acid (energetically-favorable to subsequently decarboxylate once linked on to the chain)
* the carbon that is added from CO2 is subsequently lost and not incorporated into the fatty acid
* key site of regulation
* activated by citrate (feed-forward stimulation) & insulin (via activation of a phosphatase)
* inhibited by palmitoyl-CoA (feedback inhibition) & phosphorylation via PKA (stimulated by glucagon & epinephrine) or AMPK (stimulated by high AMP levels)
* malonyl-CoA itself (a signal of lipid synthesis) is an inhibitor of CAT-1/CPT-1, the transporter that lets fatty acids into mitochondria for breakdown
* It costs 1 ATP & 2 NADPH per 2C added
* The “default” fatty acid is a 16C saturated fatty acid, palmitate, which gets cleaved off of FAS by the thioesterase subunit of FAS
* Longer fatty acids & unsaturated fatty acids can be made via elongation & desaturation in the ER
Finished in comments
 0:06:05
0:06:05
 0:05:22
0:05:22
 0:12:09
0:12:09
 0:19:46
0:19:46
 0:09:43
0:09:43
 0:17:04
0:17:04
 0:05:25
0:05:25
 0:21:53
0:21:53
 0:00:52
0:00:52
 0:12:20
0:12:20
 0:18:39
0:18:39
 0:08:49
0:08:49
 0:25:06
0:25:06
 0:05:09
0:05:09
 0:14:13
0:14:13
 0:05:04
0:05:04
 0:00:28
0:00:28
 0:06:29
0:06:29
 0:00:53
0:00:53
 0:06:05
0:06:05
 0:04:02
0:04:02
 0:11:20
0:11:20
 0:29:40
0:29:40
 0:15:26
0:15:26