filmov
tv
Using Q to Find Equilibrium Concentrations

Показать описание
This video contains the answers to the following question:
For the reaction: S(s) + O2(g) SO2(g) KC = 4.2
If we mix 2.00 M of O2 and 1.00 M of SO2 with excess S(s) what are the final concentrations of O2 and SO2?
Here is an index of the entire Video Textbook of Chemistry:
For the reaction: S(s) + O2(g) SO2(g) KC = 4.2
If we mix 2.00 M of O2 and 1.00 M of SO2 with excess S(s) what are the final concentrations of O2 and SO2?
Here is an index of the entire Video Textbook of Chemistry:
 0:04:35
0:04:35
 0:06:48
0:06:48
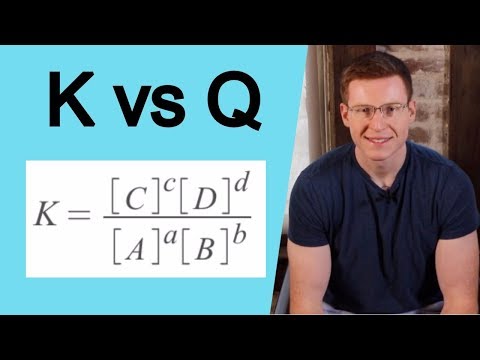 0:03:00
0:03:00
 0:10:47
0:10:47
 0:53:22
0:53:22
 0:07:27
0:07:27
 0:04:53
0:04:53
 0:05:29
0:05:29
 3:16:52
3:16:52
 0:06:08
0:06:08
 0:07:36
0:07:36
 0:06:31
0:06:31
 0:03:02
0:03:02
 0:06:15
0:06:15
 0:13:05
0:13:05
 0:09:06
0:09:06
 0:24:29
0:24:29
 0:07:50
0:07:50
 0:10:13
0:10:13
 0:10:32
0:10:32
 0:26:40
0:26:40
 0:51:39
0:51:39
 0:09:27
0:09:27
 0:43:59
0:43:59