filmov
tv
Consider the following reduction processes : `Zn^(2+)+2e^(-)toZn(s), E^(o)=-0.76 V`

Показать описание
Consider the following reduction processes : `Zn^(2+)+2e^(-)toZn(s), E^(o)=-0.76 V` `Ca^(2+)+2e^(-)toCa(s),E^(o)=-2.87 V` `Mg^(2+)+2e^(-)toMg(s),E^(o)=-2.36 V` `Ni^(2+)+2e^(-)toNi(s), E^(o)=-0.25 V The reducing power of the metals increases in the order :
 0:02:50
0:02:50
 0:02:06
0:02:06
 0:03:53
0:03:53
 0:03:53
0:03:53
 0:09:10
0:09:10
 0:00:33
0:00:33
 0:06:21
0:06:21
 0:16:05
0:16:05
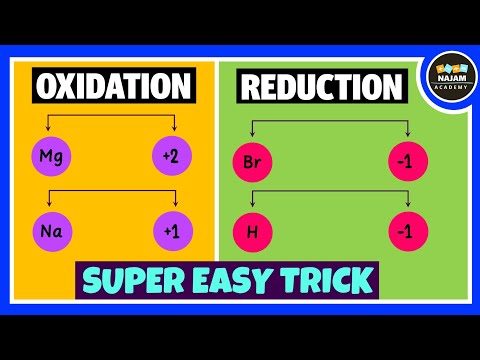 0:12:22
0:12:22
 0:10:13
0:10:13
 0:08:42
0:08:42
 0:04:53
0:04:53
 0:03:35
0:03:35
 0:16:00
0:16:00
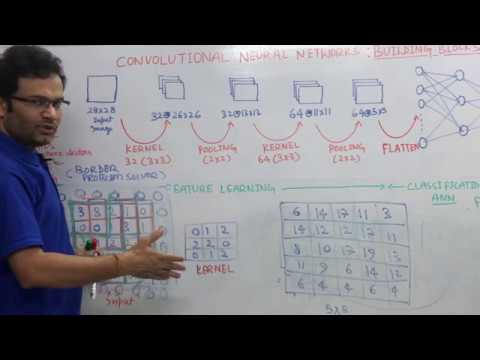 0:21:32
0:21:32
 0:05:50
0:05:50
 0:11:45
0:11:45
 0:06:51
0:06:51
 0:04:31
0:04:31
 0:13:33
0:13:33
 0:05:41
0:05:41
 0:29:19
0:29:19
 0:57:34
0:57:34
 0:06:57
0:06:57