filmov
tv
Oxidation, reduction and redox equations (AQA A level Chemistry)

Показать описание
3.1.7 Oxidation, reduction and redox equations
07:09 Determining the oxidation state of an element in a
compound or ion from the formula
27:07 Determining half-equations
38:10 Combining half- equations
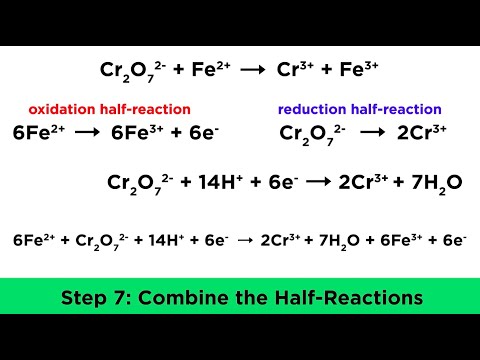
Redox reactions involve a transfer of electrons from the reducing agent to the oxidising agent. The
change in the oxidation state of an element in a compound or ion is used to identify the element that
has been oxidised or reduced in a given reaction. Separate half-equations are written for the oxidation
or reduction processes. These half-equations can then be combined to give an overall equation for any
redox reaction.
Oxidation is the process of electron loss and oxidising
agents are electron acceptors.
Reduction is the process of electron gain and reducing
agents are electron donors.
The rules for assigning oxidation states.
Students should be able to:
•• work out the oxidation state of an element in a
compound or ion from the formula
•• write half-equations identifying the oxidation and
reduction processes in redox reactions
•• combine half-equations to give an overall redox
equation.
07:09 Determining the oxidation state of an element in a
compound or ion from the formula
27:07 Determining half-equations
38:10 Combining half- equations
Redox reactions involve a transfer of electrons from the reducing agent to the oxidising agent. The
change in the oxidation state of an element in a compound or ion is used to identify the element that
has been oxidised or reduced in a given reaction. Separate half-equations are written for the oxidation
or reduction processes. These half-equations can then be combined to give an overall equation for any
redox reaction.
Oxidation is the process of electron loss and oxidising
agents are electron acceptors.
Reduction is the process of electron gain and reducing
agents are electron donors.
The rules for assigning oxidation states.
Students should be able to:
•• work out the oxidation state of an element in a
compound or ion from the formula
•• write half-equations identifying the oxidation and
reduction processes in redox reactions
•• combine half-equations to give an overall redox
equation.
Oxidation and Reduction Reactions - Basic Introduction
Introduction to Oxidation Reduction (Redox) Reactions
GCSE Chemistry - Oxidation and Reduction - Redox Reactions #39 (Higher Tier)
Oxidation-Reduction Reactions
Oxidation, Reduction, and Redox Balancing Redox Reactions
Oxidation and Reduction (Redox) Reactions Step-by-Step Example
Balancing Redox Reactions in Acidic and Basic Conditions
Oxidation and reduction | Redox reactions and electrochemistry | Chemistry | Khan Academy
Redox reactions - Chemistry - Session 17
Redox Reactions: Crash Course Chemistry #10
AQA 1.7 Oxidation, reduction and redox reactions REVISION
Redox Reactions | Explained | Full Topic | A level Chemistry
Oxidation and Reduction Reactions
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry
balancing a redox reaction / oxidation number method
Oxidation vs. Reduction, What are Oxidation and Reduction Reactions in Everyday Life?
How to Balance Redox Equations in Basic Solution
AQA A-Level Chemistry - Redox
How To Calculate Oxidation Numbers - Basic Introduction
The Oxidation Reduction Question that Tricks Everyone!
Redox Reactions
Redox Reactions | GCSE Chemistry
What is Oxidation Reduction & Redox reaction || Diffrence bw Oxidation reaction, Reduction react...
19.2 How to Balance Redox Reactions (Half-Reaction Method) | General Chemistry
Комментарии
 0:16:05
0:16:05
 0:13:05
0:13:05
 0:04:54
0:04:54
 0:03:52
0:03:52
 0:06:55
0:06:55
 0:03:56
0:03:56
 0:07:31
0:07:31
 0:11:04
0:11:04
 0:51:25
0:51:25
 0:11:13
0:11:13
 0:20:24
0:20:24
 0:26:28
0:26:28
 0:12:22
0:12:22
 0:16:00
0:16:00
 0:03:35
0:03:35
 0:05:23
0:05:23
 0:18:00
0:18:00
 0:16:22
0:16:22
 0:31:15
0:31:15
 0:06:19
0:06:19
 0:11:41
0:11:41
 0:18:59
0:18:59
 0:07:04
0:07:04
 0:32:49
0:32:49