filmov
tv
Deshaies (Amgen) 2: Cullin-RING ubiquitin ligases: structure, mechanism, and regulation
Показать описание
Part 1: A primer on the ubiquitin-proteasome system: The ubiquitin-proteasome system is one of the principal means of degrading misfolded, mutated, or unwanted proteins in the cell.
Part 2: Cullin-RING ubiquitin ligases: Over 200 types of cullin-RING ubiquitin ligases are formed by interchanging subunits. This carefully regulated system allows for great substrate specificity.
Part 3: Targeting the ubiquitin-proteasome system in cancer: The ubiquitin-proteasome system is overworked in mutation-ridden cancer cells making it a potential target for anti-cancer drugs.
Talk Overview:
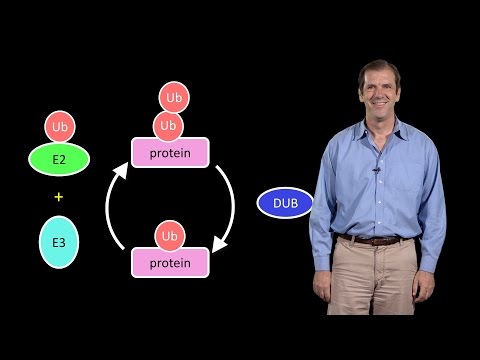
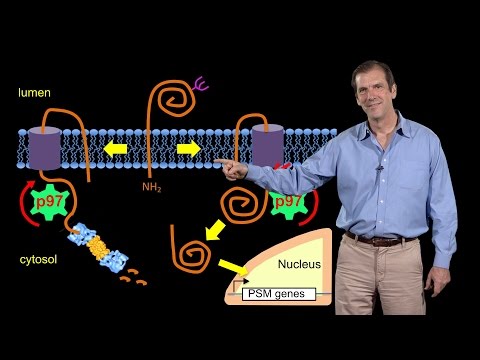
Neurons, blood cells and muscle cells have distinct morphologies and functions because they have different proteomes or collections of proteins. The proteome is maintained through the synthesis and degradation of different proteins. In his first talk, Dr. Deshaies explains that the ubiquitin-proteasome system (UPS) is the principal means of degrading proteins in the cytosol and therefore is key to maintaining the proteome. Ubiquitin is a small protein that can be added to, or removed from, other proteins altering their function or targeting them for degradation. Deshaies describes the multiple mechanisms for regulating ubiquitin ligase activity and the importance of protein degradation in many cell signaling pathways and in the removal of defective proteins.
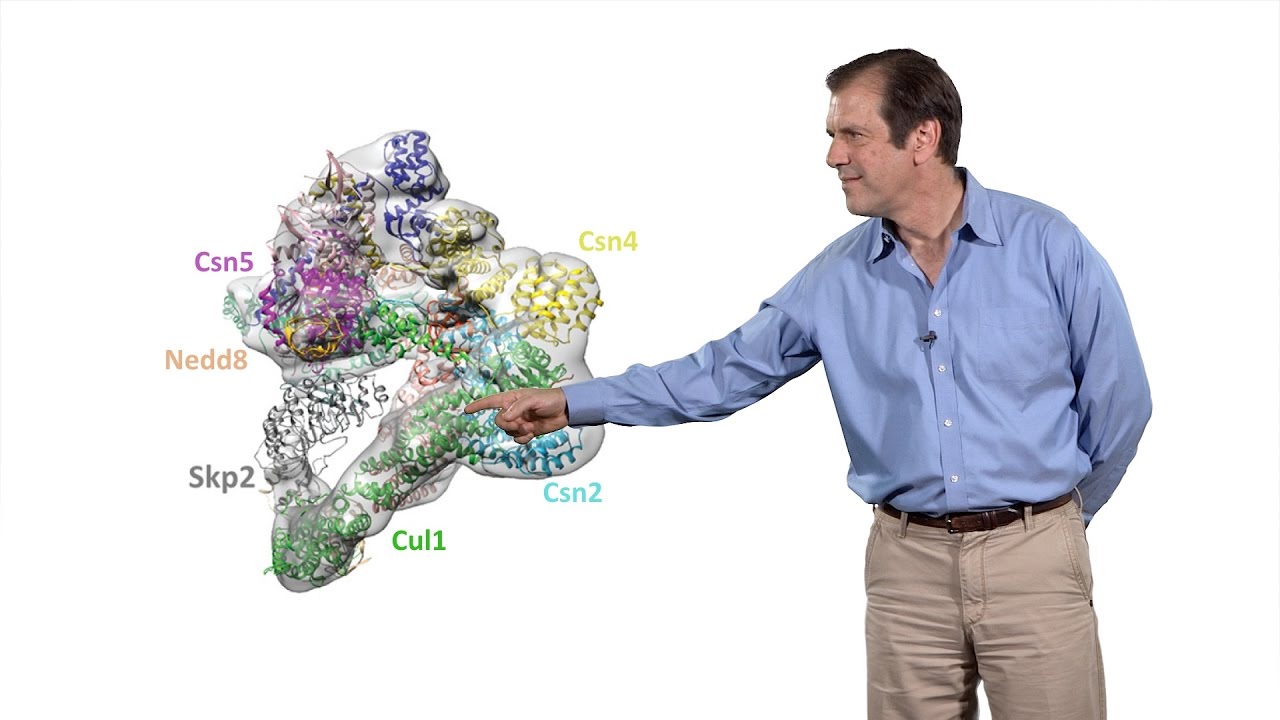
In Part 2, Deshaies delves more deeply into a specific group of ubiquitin ligases, the cullin-RING ligases (CRLs). By rearranging the subunits of CRLs in a combinatorial process, it is possible to generate more than 200 of these enzymes each with a specific substrate. The formation and activity of CRL complexes in the cell is regulated by the conjugation/deconjugation (Nedd8) or binding/dissociation (Cand1) of other proteins to the CRL complex, and this, in turn, is influenced by the binding of substrate to CRL. This carefully regulated mechanism allows cells to match the level and specificity of ubiquitin ligation to the level of particular substrates in the cell.
In many types of cancer, cells have a very high rate of mutation. These mutated proteins are frequently less stable than normal proteins and need to be degraded by the UPS. In addition, cancer cells are often aneuploid leading to the overexpression of certain proteins. These extra proteins also need to be degraded by the UPS. In both cases, the UPS in cancer cells is overworked. This suggests that cancer cells should be less able to tolerate inhibition of the UPS than normal cells. In his last talk, Deshaies describes strategies to develop cancer drugs based on inhibition of the UPS.
Speaker Biography:
At the time that this video was recorded, Dr. Raymond Deshaies was a Professor of Biology at the California Institute of Technology and an Investigator of the Howard Hughes Medical Institute. Deshaies will be transitioning to a new position as Senior Vice President for Discovery Research at Amgen, in 2017.
Deshaies received his BS in biochemistry from Cornell University and his PhD, also in biochemistry, from the University of California, Berkeley. It was as a post-doctoral fellow at the University of California, San Francisco, that Deshaies found that a cell cycle protein he was investigating was modified by the addition of ubiquitin. This began a career-long interest in the role of the ubiquitin-proteasome system in regulating protein homeostasis, both in normal cell function and in diseases such as cancer.
Deshaies has received numerous awards for his outstanding research. He has been elected as a Fellow of the American Association for the Advancement of Science (2007), as a Member of the American Academy of Arts and Sciences (2011) and as a Member of the US National Academy of Sciences (2016).
Комментарии
 0:40:35
0:40:35
 0:35:08
0:35:08
 0:41:45
0:41:45
 0:58:03
0:58:03
 0:01:56
0:01:56
 0:46:43
0:46:43
 0:49:07
0:49:07
 0:44:34
0:44:34
 0:56:43
0:56:43
 0:12:23
0:12:23
 0:53:45
0:53:45
 1:21:11
1:21:11
 0:57:15
0:57:15
 1:20:03
1:20:03
 0:09:44
0:09:44
 0:01:14
0:01:14
 0:02:36
0:02:36
 0:10:00
0:10:00
 0:07:36
0:07:36
 0:00:15
0:00:15
 0:32:51
0:32:51
 0:10:40
0:10:40
 0:00:51
0:00:51
![[Conférence] A. CIECHANOVER](https://i.ytimg.com/vi/F4Ov1k2PP-Q/hqdefault.jpg) 0:33:17
0:33:17