filmov
tv
Heating Curve and Cooling Curve of Water - Enthalpy of Fusion & Vaporization
Показать описание
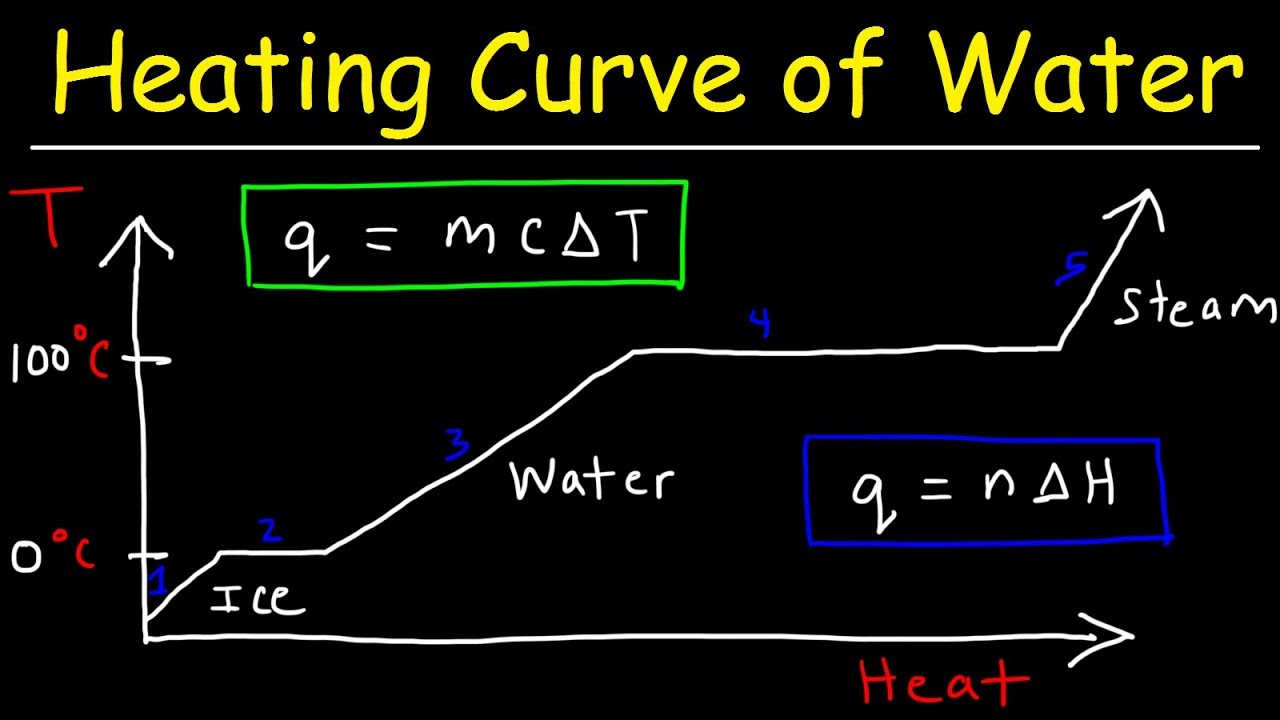
This chemistry video tutorial provides a basic introduction into the heating curve of water and the cooling curve of water. As heat is added to water, the temperature increases which increases the kinetic energy of the molecules. At the freezing point of ice, adding heat will not change the temperature but will increase the potential energy of the molecules as ice melts to liquid water. The enthalpy of fusion is the energy required to melt 1 mole of ice. The enthalpy of vaporization is the energy required to boil 1 mole of water into steam. The slope of the lines is inversely related to the specific heat capacity of the substance. The heating curve represents an endothermic process and the cooling curve is an exothermic process.
Heating Curve Chemistry Problems:
Final Temperature - Ice Water Mixture:
Molarity, Molality, Density, & Mass %:
Normality & Equivalent Weight:
_________________________________
PPM and PPB Concentrations:
How To Convert PPM to Molarity:
Enthalpy of Solution & Hydration:
Solubility Vs Concentration:
Solubility Curves:
___________________________________
Henry's Law & Gas Solubility:
Vapor Pressure & Clausius Equation:
Raoult's Law - Vapor Pressure:
Colligative Properties:
Chemical Kinetics Initial Rate Method:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Heating Curve Chemistry Problems:
Final Temperature - Ice Water Mixture:
Molarity, Molality, Density, & Mass %:
Normality & Equivalent Weight:
_________________________________
PPM and PPB Concentrations:
How To Convert PPM to Molarity:
Enthalpy of Solution & Hydration:
Solubility Vs Concentration:
Solubility Curves:
___________________________________
Henry's Law & Gas Solubility:
Vapor Pressure & Clausius Equation:
Raoult's Law - Vapor Pressure:
Colligative Properties:
Chemical Kinetics Initial Rate Method:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
 0:02:40
0:02:40
 0:13:46
0:13:46
 0:02:58
0:02:58
 0:10:43
0:10:43
 0:14:45
0:14:45
 0:08:28
0:08:28
 0:01:59
0:01:59
 0:00:59
0:00:59
 0:02:25
0:02:25
 0:18:44
0:18:44
 0:16:14
0:16:14
 0:01:11
0:01:11
 0:07:18
0:07:18
 0:12:41
0:12:41
![[4.3] Cooling and](https://i.ytimg.com/vi/tkhkaJLdesM/hqdefault.jpg) 0:01:58
0:01:58
 0:00:06
0:00:06
 0:04:26
0:04:26
 0:13:35
0:13:35
 0:01:23
0:01:23
 0:10:25
0:10:25
 0:07:22
0:07:22
 0:10:31
0:10:31
 0:01:13
0:01:13
 0:10:46
0:10:46