filmov
tv
Derivation of de broglie wavelength eqution - Physical Electronics

Показать описание
This chemistry video tutorial explains how to calculate the de broglie wavelength of large objects and small particles such as electrons. It contains plenty of examples and practice problems.
De-Broglie Wavelength
Deriving The De Broglie Wavelength
De Broglie wavelength | Physics | Khan Academy
Derive De-Broglie Wave Equation | Quantum Chemistry | Physical Chemistry
de Broglie’s proposal
De Broglie Wavelength Problems In Chemistry
Derivation of De Broglie Equation | De Broglie Wavelength #physics #cbse #neet #jeemains
The de Broglie Wavelength and Wave Particle Duality - A Level Physics
De Broglie Hypothesis | De Broglie Wavelength
De Broglie equation
Louis de Broglie's explanation of Bohr's atomic model
Derivation for De-Broglie Wavelength// Expression for De-Broglie wavelength
Derivation of de broglie wavelength eqution - Physical Electronics
Your Daily Equation #9: De Broglie Wavelength
Derivation of De Broglie Equation|De Broglie wavelength #physics #cbse #iitjee #jeemains
De Broglie wave length || chapter 8|| class 12 Physics || Tamil ||
De Broglie wavelength Derivation
Derivation of de Broglie Relationship | Atomic Structure | Chemistry |Chapter 2 | NEET | JEE
De broglie wavelength of electron | Unit 8 | 12 Physics Samacheer kalvi.
Purdue PHYS 342 L9.7: Statistical Laws of Nature: Thermal de Broglie Wavelength
Derivation of de-broglie wavelength for electron class -12th #shorts
🔥de Broglie wavelength or equation..# class12 ...# physics..#shorts ...#viral🔥
De Broglie Hypothesis | De Broglie Wavelength
The de Broglie Wavelength
Комментарии
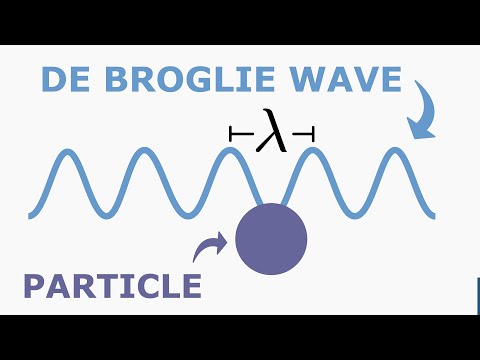 0:05:15
0:05:15
 0:03:41
0:03:41
 0:11:20
0:11:20
 0:01:43
0:01:43
 0:10:37
0:10:37
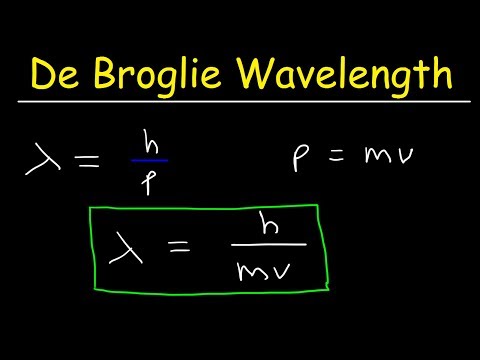 0:11:21
0:11:21
 0:00:56
0:00:56
 0:04:29
0:04:29
 0:09:05
0:09:05
 0:01:00
0:01:00
 0:08:12
0:08:12
 0:03:51
0:03:51
 0:01:50
0:01:50
 0:21:29
0:21:29
 0:00:52
0:00:52
 0:03:45
0:03:45
 0:09:43
0:09:43
 0:00:06
0:00:06
 0:04:46
0:04:46
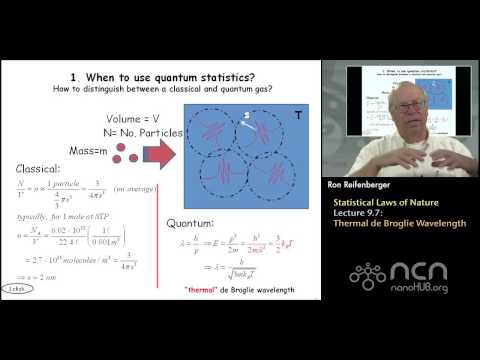 0:22:10
0:22:10
 0:00:05
0:00:05
 0:01:01
0:01:01
 0:19:00
0:19:00
 0:02:06
0:02:06