filmov
tv
Spectral Lines of Hydrogen Atom

Показать описание
This video shows the spectral lines of the hydrogen atom, represented by the orbital diagram of an atom.
AASOKA provides 2D/3D educational videos based on the CBSE curriculum that will help you understand difficult concepts and complicated experiments in a simple, easy-to-understand way. You can check all available videos of this grade and to get more clarity, or for progression of difficulty in different topics of this subject, you can check out the given playlists available on our channel:
To stay up-to-date with exciting updates about our videos and other content, connect with us on:
Please encourage us by liking our videos. Leave a comment about what we did well and how we can improve. Don't forget to subscribe to our channel to watch more educational videos!
#hydrogenatom #atoms #science #board2024
AASOKA provides 2D/3D educational videos based on the CBSE curriculum that will help you understand difficult concepts and complicated experiments in a simple, easy-to-understand way. You can check all available videos of this grade and to get more clarity, or for progression of difficulty in different topics of this subject, you can check out the given playlists available on our channel:
To stay up-to-date with exciting updates about our videos and other content, connect with us on:
Please encourage us by liking our videos. Leave a comment about what we did well and how we can improve. Don't forget to subscribe to our channel to watch more educational videos!
#hydrogenatom #atoms #science #board2024
Spectral Lines of Hydrogen Atom
Bohr Model of the Hydrogen Atom
S1.3.1 - The hydrogen emission spectrum
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
Atomic spectra | Physics | Khan Academy
Spectrum Demo: Continuous and Emission
#hydrogen vs #mecury #spectroscopy #physics #light #diffraction #science
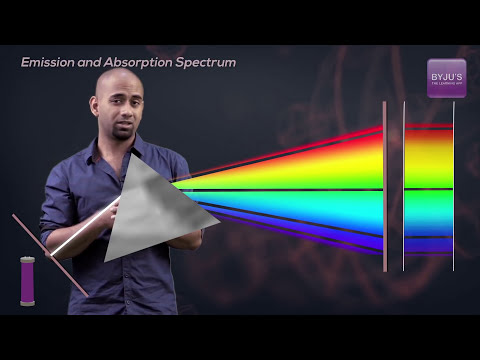
The Emission Spectrum of the Hydrogen Atom
Emission spectrum of hydrogen | Chemistry | Khan Academy
Hydrogen Spectrum
Types of Spectra | Atomic Spectra | Line Spectra | Continuous Spectra | Types of spectrum
Atomic Spectrum and Hydrogen Spectrum
Hydrogen emission spectrum class 12 | spectral lines of hydrogen atom | kpk board | punjab board
Emission Spectra and the Bohr Model
The hydrogen spectral series
Hydrogen spectrum Spectral Lines of Hydrogen Atom ViVid Chemie
Spectral series of Hydrogen atom
Hydrogen spectrum
Emission and Absorption Spectrum: JEE Chemistry Concepts Explained | Class 11th Chemistry | JEE 2023
Spectrum of Hydrogen class 12 | Atomic spectra | Atomic spectra of Hydrogen | kpk board, punjab
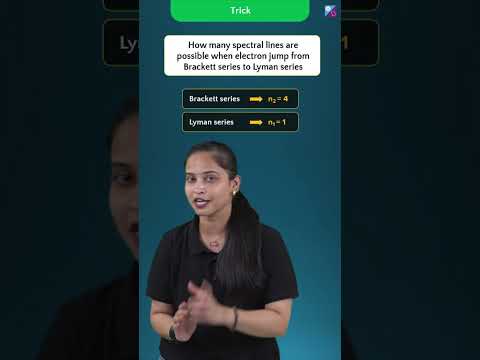
Tricks to Find Number of Spectral Lines in Hydrogen Spectrum | JEE Main & Advanced 2023/24
hydrogen spectrum #short #science #neet
#shorts #hydrogen atom,hydrogen,spectral lines of hydrogen atom,bohr model of hydrogen atom
Hydrogen Emission Spectrum Explained with Bohr Model (Atomic Absorption and Emission Spectra)
Комментарии
 0:02:14
0:02:14
 0:04:50
0:04:50
 0:08:43
0:08:43
 0:21:44
0:21:44
 0:14:43
0:14:43
 0:06:31
0:06:31
 0:00:52
0:00:52
 0:06:59
0:06:59
 0:10:50
0:10:50
 0:00:12
0:00:12
 0:02:57
0:02:57
 0:20:57
0:20:57
 0:18:03
0:18:03
 0:06:03
0:06:03
 0:24:57
0:24:57
 0:07:44
0:07:44
 0:04:26
0:04:26
 0:00:09
0:00:09
 0:03:01
0:03:01
 0:37:56
0:37:56
 0:00:43
0:00:43
 0:00:13
0:00:13
 0:00:05
0:00:05
 0:13:40
0:13:40