filmov
tv
Clinical Trials in Public Health Emergencies: the Ebola and COVID Experiences

Показать описание
Clinical Trials in Public Health Emergencies: the Ebola and COVID Experiences
Air date: Wednesday, May 13, 2020, 3:00:00 PM
Category: COVID-19
Runtime: 00:57:48
Description: NIH COVID-19 SIG Lecture Series
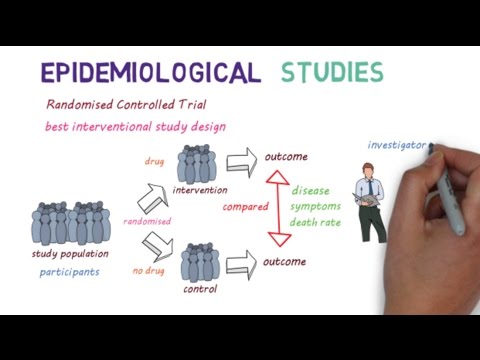
Designing and implementing clinical trials for novel infectious disease treatments brings many challenges, especially during a rapidly evolving pandemic. A new disease brings uncertainties arising from an imperfect understanding about illness, limited information about proposed countermeasures, and complexities in measuring relevant patient outcomes. A pandemic adds an overloaded medical system with limited resources for research, heightened pressure to find cures quickly, and unpredictability about potential case numbers. I will discuss issues related to designing and conducting treatment trials in outbreaks of Ebola and COVID based on my experience with three studies: Prevail II (the West African Ebola virus disease study of ZMapp), PALM (the Democratic Republic of the Congo Ebola virus disease study of ZMapp, mAb114, REGN-EB3 and remdesivir) and ACTT-1 (the multinational, platform COVID-19 study of remdesivir vs placebo).
Author: Lori Dodd, Ph.D., Mathematical Statistician, Biostatistics Research Branch, Division of Clinical Research, NIAID, NIH
Air date: Wednesday, May 13, 2020, 3:00:00 PM
Category: COVID-19
Runtime: 00:57:48
Description: NIH COVID-19 SIG Lecture Series
Designing and implementing clinical trials for novel infectious disease treatments brings many challenges, especially during a rapidly evolving pandemic. A new disease brings uncertainties arising from an imperfect understanding about illness, limited information about proposed countermeasures, and complexities in measuring relevant patient outcomes. A pandemic adds an overloaded medical system with limited resources for research, heightened pressure to find cures quickly, and unpredictability about potential case numbers. I will discuss issues related to designing and conducting treatment trials in outbreaks of Ebola and COVID based on my experience with three studies: Prevail II (the West African Ebola virus disease study of ZMapp), PALM (the Democratic Republic of the Congo Ebola virus disease study of ZMapp, mAb114, REGN-EB3 and remdesivir) and ACTT-1 (the multinational, platform COVID-19 study of remdesivir vs placebo).
Author: Lori Dodd, Ph.D., Mathematical Statistician, Biostatistics Research Branch, Division of Clinical Research, NIAID, NIH
 0:03:54
0:03:54
 0:05:23
0:05:23
 0:34:51
0:34:51
 0:31:16
0:31:16
 0:04:31
0:04:31
 0:45:13
0:45:13
 0:09:43
0:09:43
 0:28:18
0:28:18
 0:01:55
0:01:55
 0:07:33
0:07:33
 0:52:15
0:52:15
 0:02:21
0:02:21
 0:05:34
0:05:34
 0:04:20
0:04:20
 0:01:51
0:01:51
 0:01:50
0:01:50
 0:56:32
0:56:32
 0:03:50
0:03:50
 1:00:50
1:00:50
 0:07:32
0:07:32
 0:59:04
0:59:04
 0:01:14
0:01:14
 0:18:54
0:18:54