filmov
tv
Ground State vs Excited State Electron Configuration Example, Practice Problems, Explained, Summary

Показать описание
📝 Access my chemistry notes, cheat sheets, and study guides
👉 Support me on Patreon 👈
💻 Check out my highly recommended chemistry resources
In this video, we'll go over how to determine if an electron configuration is in its ground state electron configuration or excited state electron configuration. We'll start with the definition of ground state vs excited state electron configuration and then work through several examples / practice problems together.
Electron Chemistry | Ground vs Excited State
Ground State vs Excited State Electron Configuration Example, Practice Problems, Explained, Summary
Ground State vs. Excited State
5.3.1 Ground vs Excited State
Definition - Ground State
Ground State, Excited State, or Impossible Electron Configurations
Ground state vs Excited state
Ground vs Excited States
SMU vs Penn State - Round 1 CFP Final *Predictions*
Electron Configuration - Basic introduction
#6 Excited State vs Ground State
Excited and Ground State of Electrons
Where we get the terms ground state and excited state for electrons
Chapter 7: Electron Configurations of Excited States | CHM 103 | 095
Quantum Numbers, Atomic Orbitals, and Electron Configurations
K-Chem 5.7: Electron Configuration- Excited vs. Ground State
Ground State Electron Configuration | Organic Chemistry
Ground State & Excited State Including First, Second, and Third
Atomic Structure Lesson 05 Ground vs. Excited States
Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems
Excited state Meaning
Video #25 Ground vs Excited State Electron Configuration
Excited vs Ground State of Electrons
Ground state vs. excited state electron energy levels - Google Slides
Комментарии
 0:02:03
0:02:03
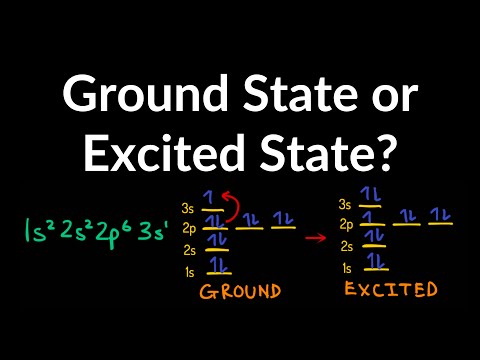 0:04:38
0:04:38
 0:05:37
0:05:37
 0:04:30
0:04:30
 0:00:06
0:00:06
 0:08:36
0:08:36
 0:02:56
0:02:56
 0:02:44
0:02:44
 0:08:57
0:08:57
 0:10:19
0:10:19
 0:02:19
0:02:19
 0:02:07
0:02:07
 0:00:39
0:00:39
 0:03:28
0:03:28
 0:08:42
0:08:42
 0:04:50
0:04:50
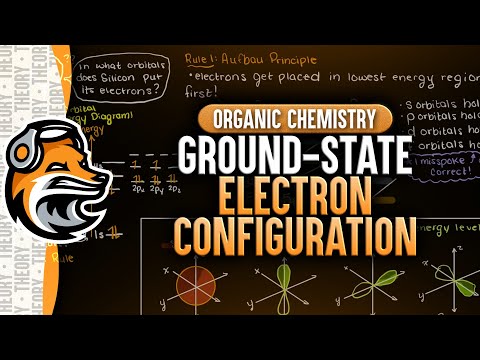 0:07:07
0:07:07
 0:05:42
0:05:42
 0:18:29
0:18:29
 0:12:12
0:12:12
 0:00:29
0:00:29
 0:07:35
0:07:35
 0:03:47
0:03:47
 0:07:14
0:07:14