filmov
tv
Balancing Chemical Equations | Law of Conservation of Mass | Chemistry 101

Показать описание
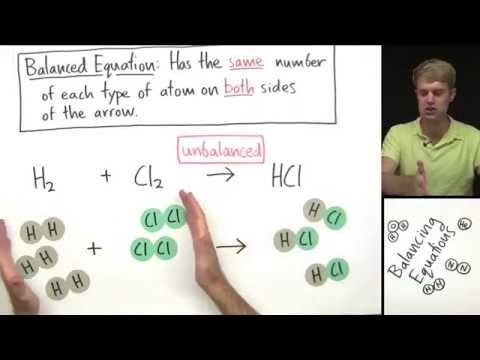
This is a basic introduction to balancing chemical equations. I go over the Law of Conservation of Mass, and why we balance equations. I show you two step-by-step examples of how to balance basic chemical equations.
Instagram: @chemistry_kelly
Twitter: @chemistry_kelly
Instagram: @chemistry_kelly
Twitter: @chemistry_kelly
How to Balance Chemical Equations in 5 Easy Steps: Balancing Equations Tutorial
Balancing Chemical Equations Practice Problems
How to Balance Chemical Equations
Balancing Chemical Equations | Law of Conservation of Mass | Chemistry 101
Balancing Chemical Equations for beginners | #aumsum #kids #science #education #children
Balancing chemical equations | Chemical reactions | High school chemistry | Khan Academy
Balancing Chemical Equations
Introduction to Balancing Chemical Equations
NEET Chemistry Past Year Trend Analysis || NEET 2025 Chemistry || @srichaitanyagosala
Balancing Chemical Equations
Balancing Chemical Equations Step by Step Practice Problems | How to Pass Chemistry
How to Balance a Chemical Equation EASY
How To Balance Chemical Equations
Balanced and Unbalanced Chemical Equations | Don't Memorise
Best Method for Balancing Chemical Equations #Chemistry #shorts #reels
Balancing Reactions Grade 10
How to Balance Chemical Equations...and Why
Balancing Chemical Equation | TAGALOG TUTORIAL!!
Balancing Chemical Equations with Polyatomic Ions
Intro to Balancing Chemical Equations
Balancing Chemical Reactions: Study Hall Chemistry #3: ASU + Crash Course
Balancing Chemical Equations Chemistry
Grade 10 - Law of Conservation of Mass and Balancing Chemical Equation, Quarter 4
How To Balance Chemical Equations? Easy Trick
Комментарии
 0:05:01
0:05:01
 0:14:56
0:14:56
 0:02:25
0:02:25
 0:06:27
0:06:27
 0:06:12
0:06:12
 0:05:03
0:05:03
 0:04:18
0:04:18
 0:12:23
0:12:23
 0:32:08
0:32:08
 0:03:00
0:03:00
 0:06:23
0:06:23
 0:08:54
0:08:54
 0:23:59
0:23:59
 0:03:54
0:03:54
 0:00:54
0:00:54
 0:17:51
0:17:51
 0:20:27
0:20:27
 0:08:50
0:08:50
 0:04:00
0:04:00
 0:47:41
0:47:41
 0:10:48
0:10:48
 0:23:48
0:23:48
 0:12:48
0:12:48
 0:14:34
0:14:34