filmov
tv
Finding Empirical And Molecular Formula Given Mass Percent Example

Показать описание
👉 Support me on Patreon 👈
💻 My highly recommended chemistry resources
HIGH SCHOOL / GENERAL CHEMISTRY
ORGANIC CHEMISTRY
In this video, you'll learn how to find the empirical and molecular formula of an unknown compound if given the mass percent of the compound. We first start by going over all the steps.
Steps
1) Change % into grams
2) Convert grams into moles by dividing grams
by molar mass
3) Divide all moles by the smallest number
4) If moles are all whole numbers, then you're done
5) If not, multiply all moles by number (usually 2 or 3)
such that everything is a whole number
Then we apply the step to an example question, going through it step by step.
By the end of the video, you'll know exactly how to find the empirical and molecular formula if given mass percent.
Empirical Formula & Molecular Formula Determination From Percent Composition
Finding and Calculating an Empirical Formula of a Compound | How to Pass Chemistry
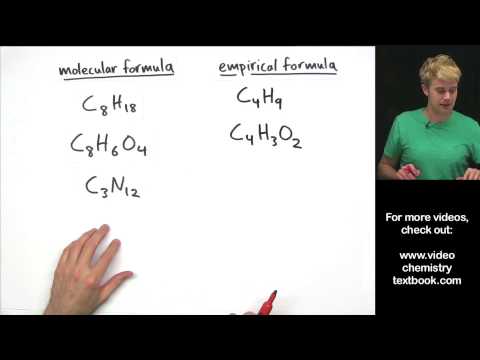
Empirical Formula and Molecular Formula Introduction
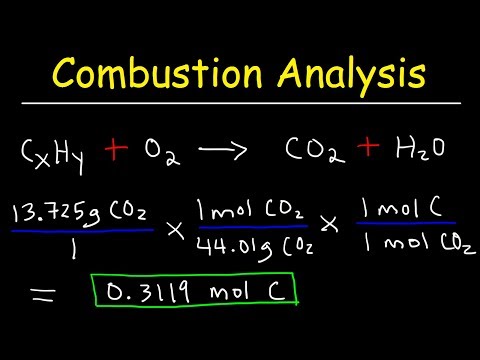
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
Calculating Molecular Formula from Empirical Formula
Practice Problem: Empirical and Molecular Formulas
Empirical and molecular formula grade 11
How to calculate Empirical Formula? 3 Easy Steps
Elemental Analysis: Empirical and Molecular Formulas
Empirical Formula and Molecular Formula | Basic Concept | Numerical Problems
Empirical and Molecular Formula from Percent Composition (No. 1)
Empirical & Molecular Formula | Good Example Question
Determining Empirical and Molecular Formulas - Chemistry Tutorial
Calculating Molecular Formulas Step by Step | How to Pass Chemistry
Calculating Molecular Formula from Empirical Formula
Writing Empirical Formula Practice Problems
How to calculate the Empirical and Molecular Formula of Compounds for 2023 JAMB tutorial
Empirical Formula | Worked Example
Finding Empirical And Molecular Formula Given Mass Percent Example
How To Calculate Empirical Formula|Super Trick|#shorts
Empirical Formulae From Percentage Composition | Chemical Calculations | Chemistry | FuseSchool
Introduction to Finding the Empirical and Molecular Formula! Exam Style Examples!
How to Find the Molecular formula from the Empirical Formula and Molar Mass
CHEMISTRY 101: Finding Empirical Formula Using Combustion Analysis for a Compound with C, H, O
Комментарии
 0:11:00
0:11:00
 0:02:52
0:02:52
 0:08:31
0:08:31
 0:16:49
0:16:49
 0:09:09
0:09:09
 0:05:53
0:05:53
 0:13:01
0:13:01
 0:05:01
0:05:01
 0:03:57
0:03:57
 0:13:30
0:13:30
 0:08:47
0:08:47
 0:00:35
0:00:35
 0:08:10
0:08:10
 0:04:26
0:04:26
 0:06:06
0:06:06
 0:06:09
0:06:09
 0:13:40
0:13:40
 0:00:33
0:00:33
 0:04:19
0:04:19
 0:00:17
0:00:17
 0:04:33
0:04:33
 0:16:44
0:16:44
 0:04:47
0:04:47
 0:04:12
0:04:12