filmov
tv
Gay-Lussac's Law (Gas Laws) - A Level Physics

Показать описание
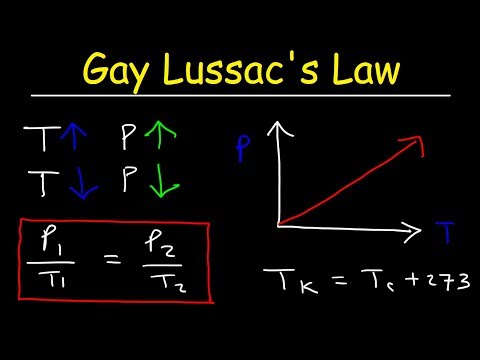
Why is it quicker to use a pressure cooker to cook your stew? It's all down to the relationship between pressure and temperature. Gay-Lussac's law is one of the 3 gas laws, telling us that the pressure of an ideal gas is proportional to temperature.
Music credit:
Song: Dipcrusher - Islands (Vlog No Copyright Music)
Music provided by Vlog No Copyright Music.
Music credit:
Song: Dipcrusher - Islands (Vlog No Copyright Music)
Music provided by Vlog No Copyright Music.
Gay-Lussac's Law (Gas Laws) - A Level Physics
Chemistry: Gay-Lussac's Law (Gas Laws) with 2 example problems
Gay lussac's Law explained(Ekdum hatke😂) #science #practical #pradi
Gay-Lussac’s law experiment
Gas Laws-Boyle's-Charles's-Gay Lussac's
Gay Lussac's Law | Gas Laws | Physical Chemistry | Khan Academy
Combined Gas Law Explained!
The ABC's of gas: Avogadro, Boyle, Charles - Brian Bennett
Feeling the Pressure of the Ideal Gas Law
The Sci Guys: Science at Home - SE2 - EP11: Gay-Lussac's Law of Ideal Gases
Gas law demonstration - Gay Lussac's law #physics #physicsexperiment #demonstration
Gay Lussac's Law Practice Problems
Chemistry: Boyle's Law (Gas Laws) with 2 example problems
Gay Lussac's Law of Gaseous Volume Explained | Class 11 Chemistry | Anupam Gupta IIT Delhi | Em...
Gas Laws in Chemistry (Boyle's, Charles', and Gay Lussac's Laws)
Gas Laws Demo #shorts #chemistry
Gay Lussac's law
3: Gas Laws
Gas Laws - Boyle’s Law, Charles, Gay-Lussac's, Avogadro Law, Ideal Gas Equation - Gas Density
Mnemonics for the Boyle, Charles, and Gay-Lussac's Law - Memorize the gas laws easily
Gay Lussac's Law | Gas Laws
Gay Lussac's law | Gas Laws | Boyle's Law | Charle's law | 11 Physics #cbse #shorts ...
gay lussac's law of gaseous volume | arvind arora chemistry | class 11 |
Gay Lussacs Law: Class X ICSE / CBSE : Gas law : Mole Concept
Комментарии
 0:02:14
0:02:14
 0:05:43
0:05:43
 0:00:43
0:00:43
 0:00:24
0:00:24
 0:02:34
0:02:34
 0:07:14
0:07:14
 0:01:00
0:01:00
 0:02:50
0:02:50
 0:00:18
0:00:18
 0:05:14
0:05:14
 0:00:20
0:00:20
 0:13:27
0:13:27
 0:05:26
0:05:26
 0:05:17
0:05:17
 0:04:46
0:04:46
 0:00:51
0:00:51
 0:00:14
0:00:14
 0:06:43
0:06:43
 0:18:55
0:18:55
 0:00:11
0:00:11
 0:03:50
0:03:50
 0:00:55
0:00:55
 0:02:49
0:02:49
 0:08:23
0:08:23