filmov
tv
How zeolites fix hard water

Показать описание
Two solutions are prepared where a few drops of 0.2 M calcium nitrate is added to distilled water. In the second solution, a zeolite is added that will exchange Na+ ions for Ca2+ ions reducing the water hardness. Upon addition of carbonate ions, the precipitate formed in the solution with zeolites is noticeably less and eventually clears up completely.
Hard water occurs when magnesium or calcium is dissolved in water and can form deposits with carbonate or with soap. Reducing the amount of calcium or magnesium ions is the goal for water softening.
Hard water occurs when magnesium or calcium is dissolved in water and can form deposits with carbonate or with soap. Reducing the amount of calcium or magnesium ions is the goal for water softening.
How zeolites fix hard water
Zeolite Method , Permutit Process for water softening process, WaterSoftening Method
Zeolite process
Hardness removal | Zeolite | Softening
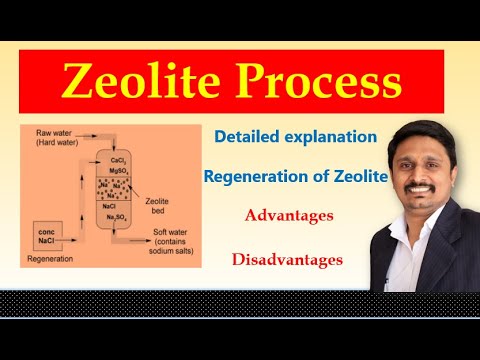
Zeolite Process for Softening of Hard water | Engineering Chemistry
How To work softing plant zeolite water 💧 hardness nill
What is Zeolite Process for Water Softening | Permutit Process | Water Softening Processes
The Power of Zeolites: An Explanation of How They Work! #pbxdetox #detox #safetyfirst #questions
Hard water treatment Zeolite Process
Fun 4 or 5 Combo Zeolite Crystal Specimen! #crystals #gems #nature #geology
Zeolite
Permutit method of softening of hard water
ZeoPad - Boiling as it should be
PBX Detox: Is it Safe? Examining the Use of Zeolite
Zeolite Process of Water Softening
Zeolites! How to detox from heavy metals, chemicals, and other harmful agents.
Zeolite Process | Permutit Process | Water Softening Process
Zeolite process
Engineering Chemistry- ZeoliteNumerical
Zeolites Explained and Simplified
Calculation of water hardness by zeolite softener
What Would You Name This Rock? Zeolites Are Fun! #crystals #nature #rocks #gems
Sodium zeolite test: The easiest way to distinguish between lithium zeolite and sodium zeolite
Zeolite Water Filtration Tank #surfacechemistry #science #cbse
Комментарии
 0:08:58
0:08:58
 0:07:30
0:07:30
 0:15:21
0:15:21
 0:06:50
0:06:50
 0:01:33
0:01:33
 0:13:29
0:13:29
 0:00:54
0:00:54
 0:12:44
0:12:44
 0:00:18
0:00:18
 0:00:35
0:00:35
 0:02:25
0:02:25
 0:00:43
0:00:43
 0:00:59
0:00:59
 0:19:07
0:19:07
 0:00:28
0:00:28
 0:12:24
0:12:24
 0:08:53
0:08:53
 0:06:18
0:06:18
 0:04:07
0:04:07
 0:18:06
0:18:06
 0:00:45
0:00:45
 0:00:19
0:00:19
 0:00:59
0:00:59