filmov
tv
Calculate Weight And Volume of Air For Coal Combustion Engineering Chemistry 2

Показать описание
Calculate the weight and volume of air required for complete combustion of 1 kg of coal containing C=65%, H=4%, O=7%, N=3%, moisture=15% and remaining is ash. (molecular weight of air=28.94 gm)
Engineering Chemistry 2 MU Question Bank Solution
#EngineeringChemistry2 #MumbaiUniversity #Combustion
Join the Telegram Group
Like Share & Subscribe to Saarang Maths
Engineering Chemistry 2 MU Question Bank Solution
#EngineeringChemistry2 #MumbaiUniversity #Combustion
Join the Telegram Group
Like Share & Subscribe to Saarang Maths
Math Antics - Volume
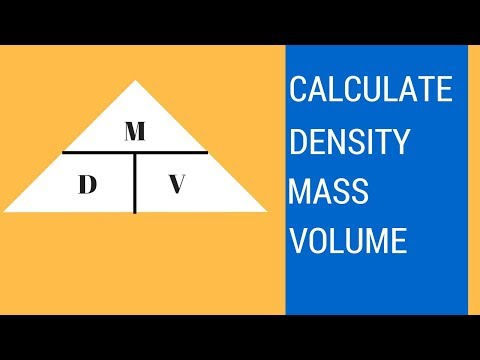
How to find density, mass, and volume
How to Calculate the Volume of a Cylinder
How to calculate weight per volume ⚡️
How to calculate percentage weight by volume (w/v)
How to Calculate the volume & weight of a concrete Cube! #shorts
The EASIEST way to calculate volumes for a weight by volume (w/v) percentage solutions
Calculate concrete cube weight in Kg
Part-2💥'Solutions : Class12 - Expressing Cocentration of Solutions- Molarity, Molalty, Mole Fra...
How To Calculate Density - With Examples
Calculate Volume, Weight, Area in CATIA V5
Calculate %v/v (Percent by Volume of a solution)
How to Calculate & Determine the Weight of a Load for Overhead Lifts
How to Calculate Gravel, Sand, & Mulch Weights and Weight per Volume | Material Estimator
Density, Mass and Volume relation? #math #tutor #physics #mathtrick #learning #shorts #youtube
Q : How to calculate Volumetric Weight?
Calculate %m/v, Mass-Volume Percent + 2 Examples
CALCULATE THE DENSITY, SPECIFIC WEIGHT, AND SPECIFIC VOLUME OF METHANE GAS
Thumb 👍 Rule ⚖️ ।। Steel bar weight calculation ⚖️।। #engineering #construction #vlog #viralvideo...
How to Calculate Precious Metal Weights in Rhino 3D CAD
Volume of a Cylinder #shorts
Weight of Different Steel Bars //10mm 12 mm 16 mm 20 mm 25 mm// #viral #shortvideo #ytshorts #shorts
How to calculate %w/v, %w/w & %v/v?
How to calculate percentage Weight by volume (%w/v). Pharmaceutical Inorganic chemistry
Комментарии
 0:12:36
0:12:36
 0:05:17
0:05:17
 0:00:31
0:00:31
 0:00:27
0:00:27
 0:00:54
0:00:54
 0:00:20
0:00:20
 0:01:53
0:01:53
 0:00:21
0:00:21
 0:50:02
0:50:02
 0:03:36
0:03:36
 0:02:51
0:02:51
 0:03:23
0:03:23
 0:08:59
0:08:59
 0:01:48
0:01:48
 0:00:30
0:00:30
 0:00:34
0:00:34
 0:03:29
0:03:29
 0:03:10
0:03:10
 0:00:05
0:00:05
 0:05:32
0:05:32
 0:00:42
0:00:42
 0:00:15
0:00:15
 0:09:11
0:09:11
 0:00:13
0:00:13