filmov
tv
How to calculate %w/v, %w/w & %v/v?

Показать описание
Explaining the concept and solving numericals related to it.
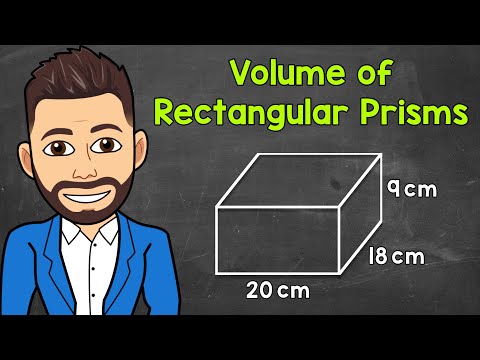
Volume of Rectangular Prisms | Math with Mr. J
How Formula 1 Brakes Work (F1 team explains)
Finding Volume with Unit Cubes | How to Find Volume
Putin and China just dealt a CRUSHING blow to the U.S. Dollar | Redacted w Clayton Morris
PAW Patrol Baby Animals Rescues & Adventures! w/ Rocky #4 🐱 10 Minutes | Nick Jr.
Why I Cheated With BLOOP In Build Battle...
Sum with Filter: Accurate totals even with filtered data in Excel?
Signing A Younger Pic With Lewis! 🥹
Bret Weinstein Makes Joe Rogan Go Quiet with Dark Election Prediction
Kamala Harris ripped after CNN town hall: 'Woefully inadequate and unprepared'
Salim Khan on his equation with Amitabh Bachchan after the split with Javed Akhtar. #shorts
Remove People in 15 Seconds with Photoshop!
Name Something Women Love That Starts With the 'D' | Family Feud
When Formula 1 collided with NBA. Thanks for stopping by, Manu 🇦🇷 #formula1 #f1 #ginobili #colapinto...
Bryan Johnson on Experimenting with his Body #shorts
Chasing Every Millisecond With TeamViewer | Find Your Better
Cool Math Trick With Rectangle And Triangle #Shorts
How I Use My Starlink Mini With My Model Y! 😤📡
Eminem Can Rhyme With ANY WORD 🐐
Gorgeous Sus Voicing with Added 3 - Jazz Piano Chord Series
Get 100 million dollars just with a code in GTA 5
English Q&A Lesson and 2,000,000 Subscribers!
Kamala Just Got STUNNED with Her WORST NEWS YET!!!
Save Money & Time with This 1 Kitchen Tool | Deals That Sing
Комментарии
 0:05:47
0:05:47
 0:15:24
0:15:24
 0:05:36
0:05:36
 0:14:06
0:14:06
 0:09:56
0:09:56
 0:22:52
0:22:52
 0:00:46
0:00:46
 0:00:19
0:00:19
 0:00:55
0:00:55
 0:09:42
0:09:42
 0:00:27
0:00:27
 0:00:15
0:00:15
 0:11:27
0:11:27
 0:00:17
0:00:17
 0:00:56
0:00:56
 0:01:01
0:01:01
 0:00:30
0:00:30
 0:00:35
0:00:35
 0:00:30
0:00:30
 0:00:13
0:00:13
 0:00:33
0:00:33
 1:00:18
1:00:18
 0:14:09
0:14:09
 0:06:15
0:06:15