filmov
tv
Colligative properties - problems

Показать описание
Table of Contents:
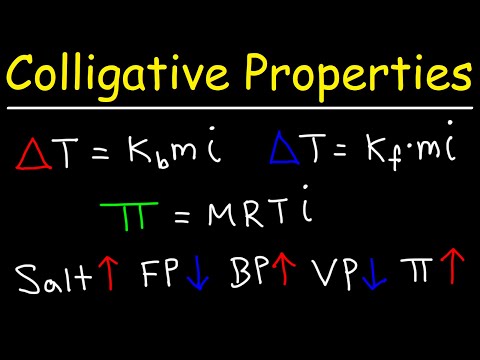
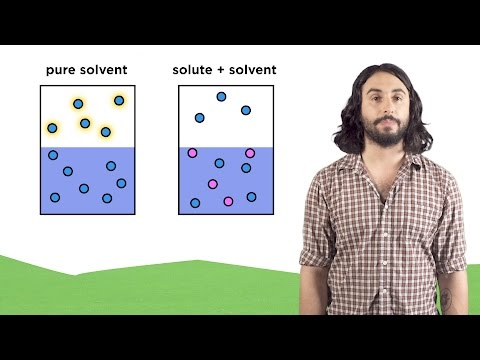
02:06 - What is the change in vapor pressure when 164 g glycerin (C3H8O3) is added to 338 mL of H2O at 39.8oC? The vapor pressure of pure H2O at 39.8oC is 54.74 torr. The density of H2O at 39.8oC is 0.992 g/mL.
04:27 - What is the change in vapor pressure when 164 g glycerin (C3H8O3) is added to 338 mL of H2O at 39.8oC? The vapor pressure of pure H2O at 39.8oC is 54.74 torr. The density of H2O at 39.8oC is 0.992 g/mL.
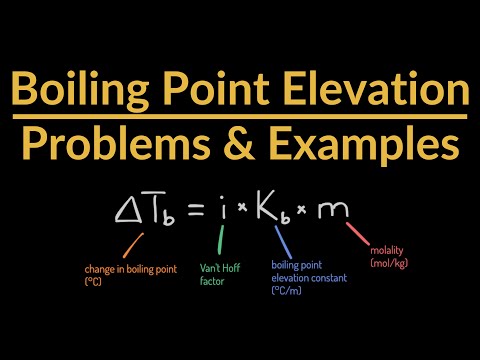
05:08 - What is the boiling point of seawater? Assume that the average concentration of salt in seawater is 0.560 mol NaCl/kilogram, the Kb value is 0.52oC/m, and that NaCl dissociates into two ions.
07:15 - What is the boiling point of seawater? Assume that the average concentration of salt in seawater is 0.560 mol NaCl/kilogram, the Kb value is 0.52oC/m, and that NaCl dissociates into two ions.
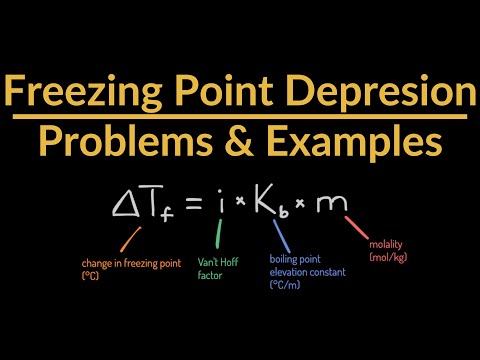
08:37 - The freezing point of a solution that contains 1.00 g of a molecular unknown compound, A, dissolved in 10.0 g of benzene is found to be 2.07oC. The freezing point of pure benzene is 5.48oC. The molal freezing-point-depression constant of benzene is 5.12oC/m. What is the molecular weight of the unknown compound?
11:24 - The freezing point depression of a 0.10 m solution of HF(aq) solution is -.201oC. Calculate the percent dissociation of HF(aq).
02:06 - What is the change in vapor pressure when 164 g glycerin (C3H8O3) is added to 338 mL of H2O at 39.8oC? The vapor pressure of pure H2O at 39.8oC is 54.74 torr. The density of H2O at 39.8oC is 0.992 g/mL.
04:27 - What is the change in vapor pressure when 164 g glycerin (C3H8O3) is added to 338 mL of H2O at 39.8oC? The vapor pressure of pure H2O at 39.8oC is 54.74 torr. The density of H2O at 39.8oC is 0.992 g/mL.
05:08 - What is the boiling point of seawater? Assume that the average concentration of salt in seawater is 0.560 mol NaCl/kilogram, the Kb value is 0.52oC/m, and that NaCl dissociates into two ions.
07:15 - What is the boiling point of seawater? Assume that the average concentration of salt in seawater is 0.560 mol NaCl/kilogram, the Kb value is 0.52oC/m, and that NaCl dissociates into two ions.
08:37 - The freezing point of a solution that contains 1.00 g of a molecular unknown compound, A, dissolved in 10.0 g of benzene is found to be 2.07oC. The freezing point of pure benzene is 5.48oC. The molal freezing-point-depression constant of benzene is 5.12oC/m. What is the molecular weight of the unknown compound?
11:24 - The freezing point depression of a 0.10 m solution of HF(aq) solution is -.201oC. Calculate the percent dissociation of HF(aq).
 0:25:23
0:25:23
 0:06:33
0:06:33
 0:29:20
0:29:20
 0:15:59
0:15:59
 0:05:10
0:05:10
 0:34:57
0:34:57
 0:10:07
0:10:07
 0:04:41
0:04:41
 0:50:56
0:50:56
 0:15:32
0:15:32
 0:31:16
0:31:16
 0:09:52
0:09:52
 0:15:32
0:15:32
 0:15:45
0:15:45
 0:10:59
0:10:59
 0:14:02
0:14:02
 1:08:00
1:08:00
 0:48:51
0:48:51
 0:18:45
0:18:45
 0:10:31
0:10:31
 0:16:08
0:16:08
 0:12:13
0:12:13
 0:20:49
0:20:49
 1:14:49
1:14:49