filmov
tv
Biosignaling | Receptor Tyrosine Kinase (RTKs) Autophosphorylation [Part 1/2]

Показать описание
Welcome to Catalyst University! I am Kevin Tokoph, PT, DPT, and this is one of my earlier biochemistry videos where we discuss receptor tyrosine kinases (RTKs) and how they perform auto-phosphorylation.
Please leave a like and subscribe! 🙏
INSTAGRAM | @thecatalystuniversity
Follow me on Instagram @thecatalystuniversity for additional helpful content and for my more fun side: Pets, Workouts, Dragon Ball Z
MERCHANDISE
Be sure to check out custom Catalyst University merchandise!
PATREON
Please leave a like and subscribe! 🙏
INSTAGRAM | @thecatalystuniversity
Follow me on Instagram @thecatalystuniversity for additional helpful content and for my more fun side: Pets, Workouts, Dragon Ball Z
MERCHANDISE
Be sure to check out custom Catalyst University merchandise!
PATREON
Biosignaling | Receptor Tyrosine Kinases & Insulin
Biosignaling | Receptor Tyrosine Kinase (RTKs) Autophosphorylation [Part 1/2]
Biosignaling | Receptor Tyrosine Kinases (RTKs) [Part 1/2]
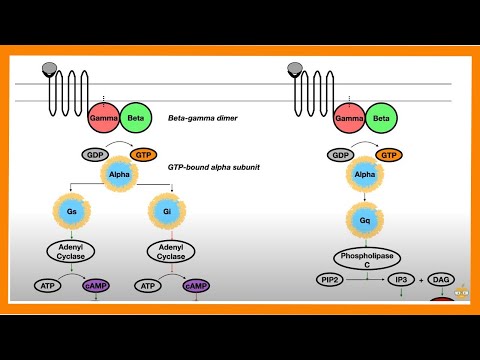
Common cell signaling pathway
Signal Transduction Pathways (G-Protein, Receptor Tyrosine Kinase, cGMP)
Insulin Receptor Tyrosine Kinase (RTK) - How insulin works - Diabetes Mellitus - Sugar (Glucose)
Schneid Guide to Receptor Tyrosine Kinases
JAK-STAT Pathway — Non-receptor Tyrosine Kinase (NRTK) - Cell Signaling - Endocrinology Playlist
Receptor Tyrosine Kinases
Receptor Tyrosine Kinase || MAPK Pathway
Enzyme Linked Receptors | Nervous system physiology | NCLEX-RN | Khan Academy
Receptor Tyrosine Kinases
Receptor Tyrosine Kinases - Structure & Functions in Signal Transduction - Simply Explained..!!!
Receptor Tyrosine Kinase
RECEPTOR TYROSINE KINASES
Endocrinology | Receptor Pathways
Lecture 08, concept 19: Signaling - Receptor Tyrosine Kinases (RTKs)
Tyrosine Kinase Receptor and Non Tyrosine Kinase Receptors
Hormones: Receptor Tyrosine Kinase - Bio-Signaling 07
Cell Signaling- Tyrosine Kinase receptors
G Protein Coupled Receptors and Receptor Tyrosine Kinases
Receptor Tyrosin Kinase (RTK) Pharmacology
5-6 Receptor Tyrosine Kinases
Biosignaling - GPCRs (including adrenergic receptors) & RTKs (Receptor Tyrosine Kinases)
Комментарии
 0:11:05
0:11:05
 0:19:09
0:19:09
 0:19:42
0:19:42
 0:09:41
0:09:41
 0:17:26
0:17:26
 0:13:47
0:13:47
 0:11:51
0:11:51
 0:07:56
0:07:56
 0:06:39
0:06:39
 0:07:26
0:07:26
 0:08:51
0:08:51
 0:13:09
0:13:09
 0:04:13
0:04:13
 0:03:54
0:03:54
 0:01:19
0:01:19
 0:28:04
0:28:04
 0:04:26
0:04:26
 0:02:34
0:02:34
 0:26:52
0:26:52
 0:05:34
0:05:34
 0:06:25
0:06:25
 0:01:59
0:01:59
 0:05:41
0:05:41
 1:28:42
1:28:42