filmov
tv
Using the Nernst Equation to calculate the Cell Potential at NON-standard conditions

Показать описание
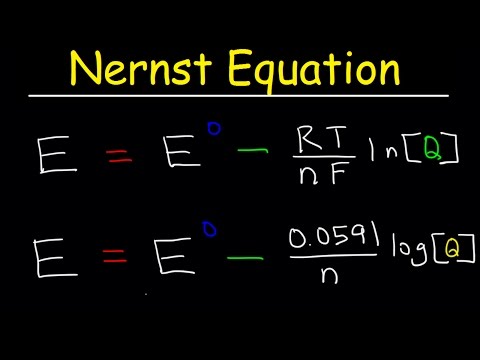
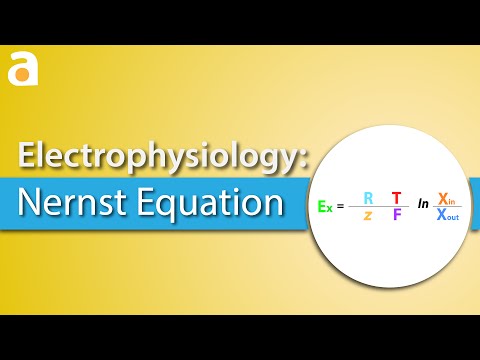
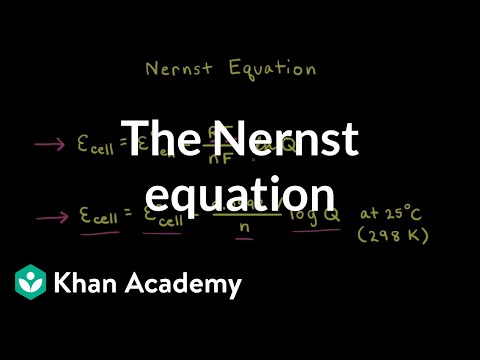
This video takes the Gibbs free energy equation at non-standard conditions and makes an algebraic substitution to derive the Nernst Equation which is used in Electrochemical Cell potential calculations when concentrations are NOT 1.0 Molar. We can use the Nernst equation to determine an equilibrium constant, K.
 0:30:53
0:30:53
 0:03:25
0:03:25
 0:04:07
0:04:07
 0:11:30
0:11:30
 0:11:27
0:11:27
 0:10:31
0:10:31
 0:06:57
0:06:57
 0:07:50
0:07:50
 0:00:32
0:00:32
 0:13:27
0:13:27
 0:18:28
0:18:28
 0:25:14
0:25:14
 0:22:25
0:22:25
 0:11:05
0:11:05
 0:02:32
0:02:32
 0:10:42
0:10:42
 0:10:50
0:10:50
 0:00:59
0:00:59
 0:14:45
0:14:45
 0:09:46
0:09:46
 0:06:38
0:06:38
 0:10:58
0:10:58
 0:12:06
0:12:06
 0:07:48
0:07:48