filmov
tv
Using the Nernst Equation to Calculate Cell Potential (Ecell) 003

Показать описание
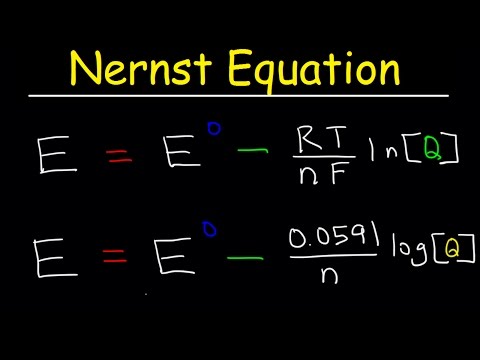
Calculate the cell potential (Ecell) at 25°C for the reaction:
2 Al (s) + 3 Fe2+ (aq) → 2 Al3+ (aq) + 3 Fe (s)
given that [Fe2+] = 0.020 M, [Al3+] = 0.10 M, and the standard reduction potential is -1.66 V for Al3+/Al and -0.45 V for Fe2+/Fe.
2 Al (s) + 3 Fe2+ (aq) → 2 Al3+ (aq) + 3 Fe (s)
given that [Fe2+] = 0.020 M, [Al3+] = 0.10 M, and the standard reduction potential is -1.66 V for Al3+/Al and -0.45 V for Fe2+/Fe.
 0:30:53
0:30:53
 0:03:25
0:03:25
 0:04:07
0:04:07
 0:11:30
0:11:30
 0:11:27
0:11:27
 0:10:31
0:10:31
 0:06:57
0:06:57
 0:07:50
0:07:50
 0:09:04
0:09:04
 0:13:27
0:13:27
 0:18:28
0:18:28
 0:25:14
0:25:14
 0:11:05
0:11:05
 0:22:25
0:22:25
 0:02:32
0:02:32
 0:10:42
0:10:42
 0:10:50
0:10:50
 0:09:46
0:09:46
 0:00:59
0:00:59
 0:06:38
0:06:38
 0:10:58
0:10:58
 0:12:06
0:12:06
 0:07:48
0:07:48
 0:12:20
0:12:20