filmov
tv
Understand SDS-PAGE In Under 4 Minutes

Показать описание
0:00-1:20 | What is SDS-PAGE?
1:20-3:24 | How does SDS-PAGE work?
3:24-4:17 | What are protein ladders?
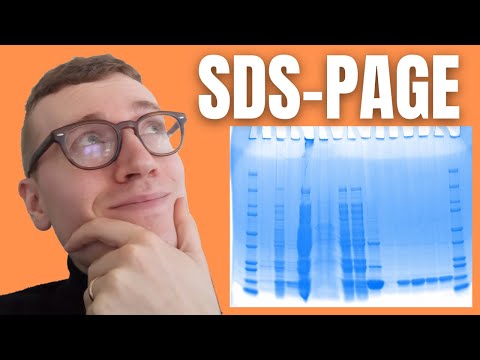
SDS-PAGE stands for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and is one of the most widely used types of protein electrophoresis. Protein electrophoresis is a standard laboratory technique during which charged protein molecules are transported through a solvent by an electrical field. Both proteins and nucleic acids may be separated by electrophoresis. Biological molecules tend to carry a net charge, at any pH other than their isoelectric point, meaning that they will migrate towards a positive charge. (I have a video explaining the isoelectric point in greater detail which I’ll link by the end of this video) The mobility of a molecule through such an electric field will depend on the field strength of the field, net charge on the molecule as well as its size and shape in addition to the ionic strength and the properties of the matrix. Polyacrylamide has smaller pores making it ideal for separating the majority of proteins in addition to smaller nucleic acids.
SDS-PAGE separates proteins primarily by mass because the ionic detergent SDS denatures and binds to proteins to make them uniformly negatively charged. This is essential since there is only one more factor affecting protein movement. Therefore, when a current is applied, all SDS-bound proteins in the sample move through the gel towards the positively charged electrode. Smaller proteins, or in other words, proteins with lesser mass move quicker compared to those with greater mass due to how the matrix is constructed. For our purposes, think of it as several fishing nets lined up after each other that still give way. You can imagine that in this scenario, small fishes can move relatively unrestricted while big fishes get slowed down much more by these fishing nets.
When the samples have been separated by electrophoresis, i.e. when we have applied an electric field over the gel for a specific amount of time, we can compare the various stains with standardized ladders to determine the relative sizes of our samples. The separated samples can also be transferred for further separation, using for example isoelectric focusing, and/or extracted fir further analysis by mass spectrometry (isoelectric focusing is also something I have previously covered so I will also link that by the end of this video). For these reasons, gel electrophoresis is fundamental in many kinds of proteomic analyses.
To asses molecular masses, i.e. the sizes of proteins in a gel, a prepared mixture containing several proteins of known molecular masses is run alongside the test sample in one or more lanes of the gel. These sets are called molecular weight markers or more commonly, at least in my somewhat limited experience, protein ladders. One can construct a standard curve from the distances traveled by each of these markers and from this standard curve, one can extrapolate the molecular weights of our samples.
1:20-3:24 | How does SDS-PAGE work?
3:24-4:17 | What are protein ladders?
SDS-PAGE stands for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and is one of the most widely used types of protein electrophoresis. Protein electrophoresis is a standard laboratory technique during which charged protein molecules are transported through a solvent by an electrical field. Both proteins and nucleic acids may be separated by electrophoresis. Biological molecules tend to carry a net charge, at any pH other than their isoelectric point, meaning that they will migrate towards a positive charge. (I have a video explaining the isoelectric point in greater detail which I’ll link by the end of this video) The mobility of a molecule through such an electric field will depend on the field strength of the field, net charge on the molecule as well as its size and shape in addition to the ionic strength and the properties of the matrix. Polyacrylamide has smaller pores making it ideal for separating the majority of proteins in addition to smaller nucleic acids.
SDS-PAGE separates proteins primarily by mass because the ionic detergent SDS denatures and binds to proteins to make them uniformly negatively charged. This is essential since there is only one more factor affecting protein movement. Therefore, when a current is applied, all SDS-bound proteins in the sample move through the gel towards the positively charged electrode. Smaller proteins, or in other words, proteins with lesser mass move quicker compared to those with greater mass due to how the matrix is constructed. For our purposes, think of it as several fishing nets lined up after each other that still give way. You can imagine that in this scenario, small fishes can move relatively unrestricted while big fishes get slowed down much more by these fishing nets.
When the samples have been separated by electrophoresis, i.e. when we have applied an electric field over the gel for a specific amount of time, we can compare the various stains with standardized ladders to determine the relative sizes of our samples. The separated samples can also be transferred for further separation, using for example isoelectric focusing, and/or extracted fir further analysis by mass spectrometry (isoelectric focusing is also something I have previously covered so I will also link that by the end of this video). For these reasons, gel electrophoresis is fundamental in many kinds of proteomic analyses.
To asses molecular masses, i.e. the sizes of proteins in a gel, a prepared mixture containing several proteins of known molecular masses is run alongside the test sample in one or more lanes of the gel. These sets are called molecular weight markers or more commonly, at least in my somewhat limited experience, protein ladders. One can construct a standard curve from the distances traveled by each of these markers and from this standard curve, one can extrapolate the molecular weights of our samples.
Комментарии
 0:04:17
0:04:17
 0:07:55
0:07:55
 0:09:37
0:09:37
 0:05:29
0:05:29
 0:01:26
0:01:26
 0:02:04
0:02:04
 0:04:23
0:04:23
 0:00:16
0:00:16
 0:17:28
0:17:28
 0:07:34
0:07:34
 0:04:51
0:04:51
 0:04:49
0:04:49
 0:09:39
0:09:39
 0:01:13
0:01:13
 0:11:44
0:11:44
 0:03:32
0:03:32
 0:00:34
0:00:34
 0:02:44
0:02:44
 0:06:53
0:06:53
 0:05:33
0:05:33
 0:04:44
0:04:44
 0:04:23
0:04:23
 0:24:16
0:24:16
 0:01:38
0:01:38