filmov
tv
What Is Buffer Capacity?

Показать описание
This video discusses the definition of buffer capacity and how to use that definition to answer questions related to comparing the buffer capacity of various solutions.
What Is Buffer Capacity?
Buffer capacity | Buffers, titrations, and solubility equilibria | Chemistry | Khan Academy
Buffer capacity | Acids and bases | AP Chemistry | Khan Academy
27 Buffer Capacity and pH Range
Buffers: Capacity & Ranges – Chemistry | Lecturio
Buffers and Buffer Capacity Demonstration
What is Buffer Capacity? and what is the condition for maximum buffer capacity?
How Buffers Work and Buffer Capacity
How do you calculate buffer capacity?
What is a Buffer?
Buffers, the Acid Rain Slayer: Crash Course Chemistry #31
Acid-Base Equilibria and Buffer Solutions
Buffer Solutions
BUFFER CAPACITY
pH and Buffers
Buffer capacity
Buffer Solutions Explained Simply: What is a Buffer and How Does a Buffer Solution Work?
Exploring Buffers and Buffer Capacity | Intro & Theory
Buffer Capacity | CHEMISTRY | JEE | Concept of the Day | SM Sir
Buffer solutions- pH range and buffering capacity
Bicarbonate Buffer System
Buffer range | Acids and bases | AP Chemistry | Khan Academy
2 Minute Turf Talk - Buffering Capacity
Physiologic pH and buffers - acid-base physiology
Комментарии
 0:02:25
0:02:25
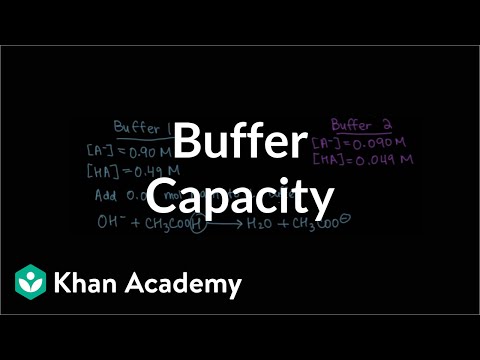 0:10:44
0:10:44
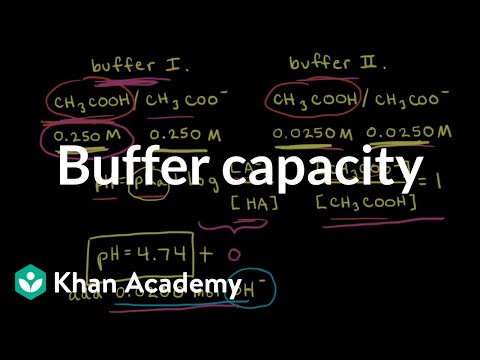 0:10:18
0:10:18
 0:02:54
0:02:54
 0:04:17
0:04:17
 0:04:39
0:04:39
 0:04:54
0:04:54
 0:14:20
0:14:20
 0:06:47
0:06:47
 0:04:27
0:04:27
 0:11:41
0:11:41
 0:05:04
0:05:04
 0:33:21
0:33:21
 0:05:29
0:05:29
 0:05:57
0:05:57
 0:05:56
0:05:56
 0:07:31
0:07:31
 0:19:15
0:19:15
 0:08:52
0:08:52
 0:20:06
0:20:06
 0:06:28
0:06:28
 0:05:17
0:05:17
 0:02:02
0:02:02
 0:10:31
0:10:31