filmov
tv
Dalton’s Atomic Theory | Don't Memorise

Показать описание
In this video, we will learn:
0:00 Introduction
0:25 Dalton's atomic theory
0:51 postulates of Dalton's atomic theory
1:44 all matter is made up of very tiny particles called atoms
2:17 atoms are indivisible particles which cannot be created or destroyed in a chemical reaction
3:02 atoms of a given element are identical in mass and chemical properties
3:38 atoms of different elements have different masses and chemical properties
4:07 atoms combine in a ratio of small whole numbers to form compounds
5:11 the relative number and kinds of atoms are constant in a given compound
Register on our website to gain access to all videos and quizzes:
#DaltonsAtomicTheory #AtomicTheory #InfinityLearn #neet2024 #infinityLearnNEET #neetsyllabus #neet2025
Dalton’s Atomic Theory | Don't Memorise
Dalton's Atomic Theory
Dalton's Atomic Theory
Dalton's Atomic Theory | #aumsum #kids #science #education #children
Why Did Dalton's Atomic Theory Fail? | Explained in less than 2 minutes | By Cerebroz EduTree
History of Atomic Theory
Dalton's Atomic Theory || 3D Animated explanation || Complete Basics || Chemistry || Class 9th ...
Dalton atomic theory
Dalton’s Atomic Model | Science and Technology | Alloprof
Dalton's Atomic Theory | Sanjay Arya IIT | Chemistry Expert | Chemistry | JEE | Embibe: Achieve...
CHEMISTRY 101: The three laws that led to Daltons Atomic Theory
Dalton's Atomic Theory
John Dalton Biography | Animated Video | Discovered the Atomic Theory
How small are atoms?
Atoms » Dalton’s Atomic Theory & Limitations
Daltons Atomic Theory | Physical Chemistry | NEET JEE | ATP STAR
Dalton's Atomic Theory
Dalton's atomic theory #shorts
Bohr’s Atomic Model | Atoms and Molecules | Infinity Learn NEET
Dalton's Atomic theory- Explained
Last Words of Albert Einstein #shorts
Dalton's Atomic Theory | General Chemistry I | 007
Dalton's atomic theory.#daltons #theory
Dalton's Atomic Theory, 1808
Комментарии
 0:06:48
0:06:48
 0:06:27
0:06:27
 0:04:02
0:04:02
 0:05:20
0:05:20
 0:01:12
0:01:12
 0:04:26
0:04:26
 0:02:52
0:02:52
 0:07:24
0:07:24
 0:03:48
0:03:48
 0:13:22
0:13:22
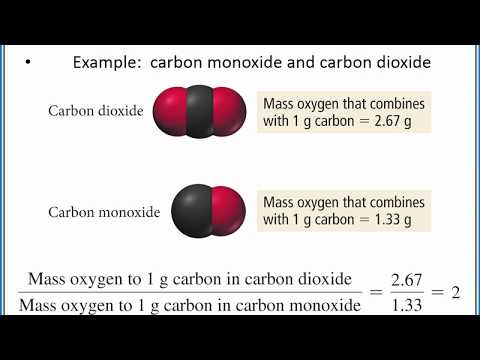 0:04:06
0:04:06
 0:04:55
0:04:55
 0:08:57
0:08:57
 0:00:48
0:00:48
 0:12:26
0:12:26
 0:08:49
0:08:49
 0:05:32
0:05:32
 0:00:38
0:00:38
 0:05:05
0:05:05
 0:01:17
0:01:17
 0:00:37
0:00:37
 0:08:37
0:08:37
 0:00:12
0:00:12
 0:08:11
0:08:11