filmov
tv
Aldol condensation | Alpha Carbon Chemistry | Organic chemistry | Khan Academy
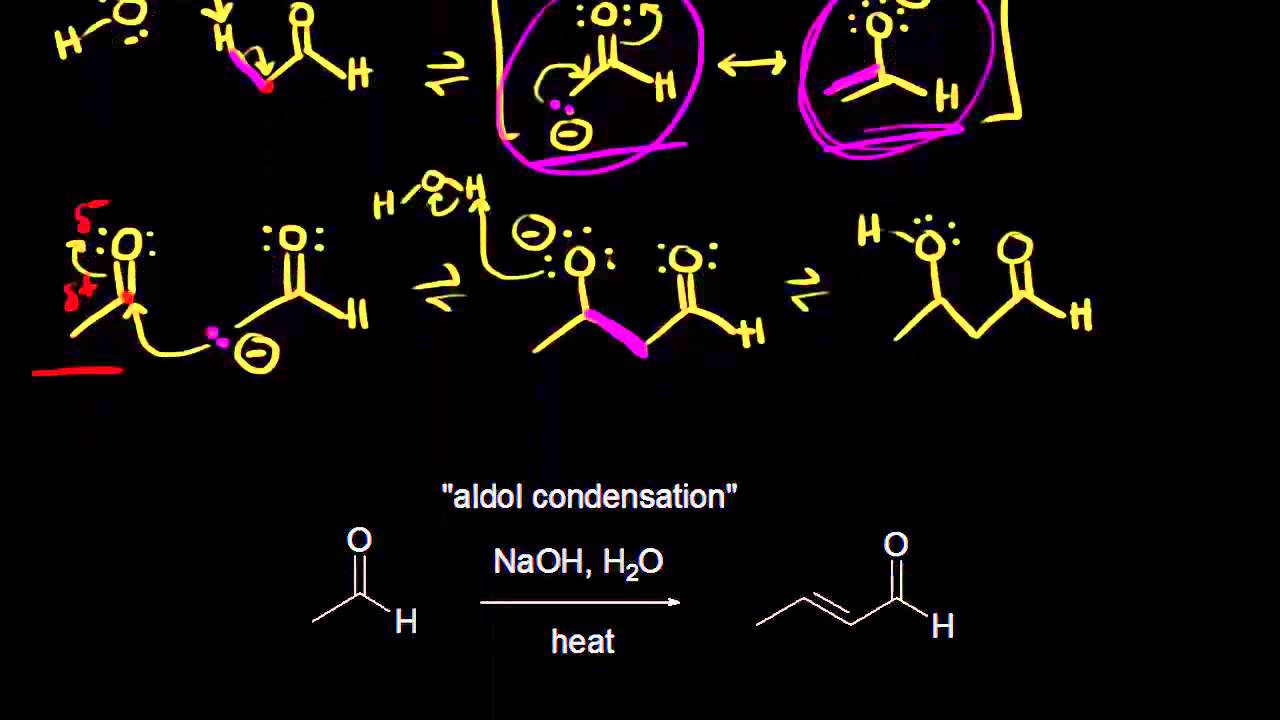
Показать описание
Base-catalyzed mechanism for aldol addition and aldol condensation. Created by Jay.
Organic Chemistry on Khan Academy: Carbon can form covalent bonds with itself and other elements to create a mind-boggling array of structures. In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them. We will also think about how those reactions are occurring on a molecular level with reaction mechanisms. Simply put, organic chemistry is like building with molecular Legos. Let's make some beautiful organic molecules!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Organic Chemistry on Khan Academy: Carbon can form covalent bonds with itself and other elements to create a mind-boggling array of structures. In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them. We will also think about how those reactions are occurring on a molecular level with reaction mechanisms. Simply put, organic chemistry is like building with molecular Legos. Let's make some beautiful organic molecules!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Комментарии
 0:05:00
0:05:00
 0:11:23
0:11:23
 0:22:22
0:22:22
 0:11:41
0:11:41
 0:08:04
0:08:04
 0:12:35
0:12:35
 0:09:25
0:09:25
 0:12:02
0:12:02
 0:11:13
0:11:13
 0:02:36
0:02:36
 0:11:36
0:11:36
 0:05:24
0:05:24
 0:10:37
0:10:37
 0:11:14
0:11:14
 0:03:25
0:03:25
 0:15:14
0:15:14
 0:04:37
0:04:37
 0:13:11
0:13:11
 0:11:29
0:11:29
 0:03:08
0:03:08
 0:03:32
0:03:32
 0:32:18
0:32:18
 0:00:14
0:00:14
 0:09:02
0:09:02