filmov
tv
C5 CO2 and the Sea (equilibria) [SL IB Chemistry]

Показать описание
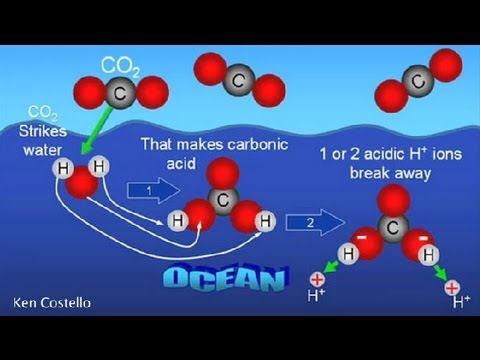
CO2 dissolves into the sea to form carbonic acid. Acids lower the pH of the ocean and dissolve coral. This is bad.
C5 CO2 and the Sea (equilibria) [SL IB Chemistry]
Carbonate Buffering
How Does Carbon Dioxide Enter Sea Water? : Earth Science
DIC The Carbonate System In the Ocean
The Truth about CO2 and Sea Level Rise - Alex Epstein Q&A at CMU
S3.2.9 Why are CO2 and H2O Such Good Greenhouse Gases? [HL IB Chemistry]
The Chemistry of Ocean Acidification
IB Chemistry HL&SL | Option C: Energy | C.5 - Environmental Impact: Global Warming
Oceans removing more CO2 than Scientist Expected
CHM131 - OCEAN ACIDIFICATION
Sea, Satellites and CO2
An Introduction to Ocean Acidification
Background Chemistry for Ocean Acidification (Gasses and Climate)
why and how do oceans absorb carbon dioxide?
C5 Global Warming Overview [SL IB Chemistry]
C5.1 - Greenhouse
AE#10 CO2 solubility
Ocean acidification explained in two minutes
Environmental Chemistry Chapter 6 Lesson 3: Carbonates and Ocean Acidification
Oceans, Carbon and Heat. Have we wrecked another equilibrium?
How does ocean acidification relate to equilibrium?
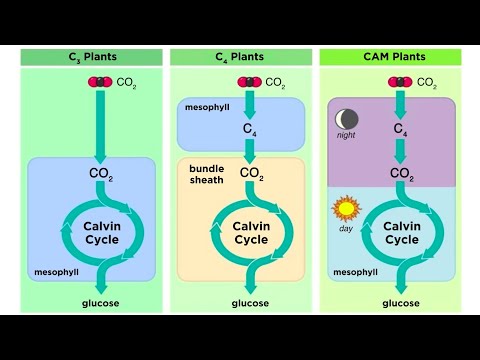
Types of Photosynthesis in Plants: C3, C4, and CAM
Acids08 Equilibrium and CO2
Equilibrium and Ocean Acidification
Комментарии
 0:04:43
0:04:43
 0:03:22
0:03:22
 0:03:12
0:03:12
 0:18:58
0:18:58
 0:04:20
0:04:20
 0:02:23
0:02:23
 0:00:34
0:00:34
 0:22:00
0:22:00
 0:02:21
0:02:21
 0:07:26
0:07:26
 0:06:06
0:06:06
 0:08:19
0:08:19
 0:10:54
0:10:54
 0:01:55
0:01:55
 0:04:55
0:04:55
 0:28:25
0:28:25
 0:06:42
0:06:42
 0:02:20
0:02:20
 0:37:18
0:37:18
 0:12:05
0:12:05
 0:02:40
0:02:40
 0:06:51
0:06:51
 0:11:46
0:11:46
 0:02:57
0:02:57