filmov
tv
Adiabatic Process kya hoti hai || What is Adiabatic process

Показать описание
Free Demo Course of All in 1 AE JE
For SSC JE, RRB JE, HPCL, NHPC, ISRO
Click Here for free course
Click here to download our app
Join telegram channel
SSC JE / RRB JE / ISRO / NHPC / SAIL / TATA Junior Engineer
की तयारी के लिए App Download करें
or visit our website
Join our whats app channel
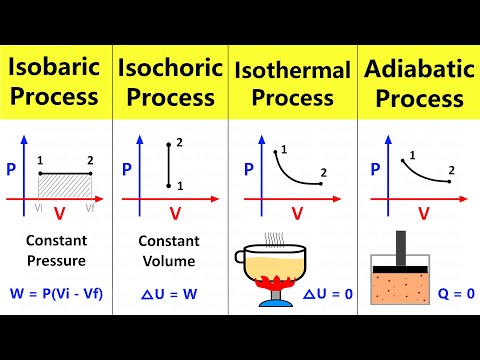
What is Adiabatic process? The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. An adiabatic process is a reversible process with constant entropy for an ideal gas. The mathematical representation of the adiabatic process is ΔQ=0.
What is the difference between isolated system and adiabatic system?
Re: Isolated vs. Adiabatic system. The internal energy of an isolated system will not change over time. In an adiabatic system, energy is not transferred as heat, but the internal energy can still change if energy is transferred to or from the system as work.
What is an adiabatic process with an example?
Adiabatic Process Examples
In an engine, gasoline is mixed with air and then adiabatically compressed, causing its temperature to increases rapidly and dramatically. Then, a spark plug ignites the air-gasoline mixture in the cylinder. It then undergoes another adiabatic process as the gas in the cylinder expands.
What is an adiabatic system?
An adiabatic process is defined as a process in which no heat transfer takes place. This does not mean that the temperature is constant, but rather that no heat is transferred into or out from the system
What are the types of adiabatic?
Adiabatic Process - Definition, Equation, Reversible ...
The thermodynamic process in which there is no exchange of heat from the system to its surrounding neither during expansion nor during compression. The adiabatic process can be either reversible or irreversible.
Adiabatic process
Adiabatic process formula
Adiabatic system in thermodynamics pdf
Adiabatic process example
Adiabatic system in thermodynamics notes
Adiabatic system in thermodynamics formula
Adiabatic system in thermodynamics wikipedia
Adiabatic process Derivation
For SSC JE, RRB JE, HPCL, NHPC, ISRO
Click Here for free course
Click here to download our app
Join telegram channel
SSC JE / RRB JE / ISRO / NHPC / SAIL / TATA Junior Engineer
की तयारी के लिए App Download करें
or visit our website
Join our whats app channel
What is Adiabatic process? The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. An adiabatic process is a reversible process with constant entropy for an ideal gas. The mathematical representation of the adiabatic process is ΔQ=0.
What is the difference between isolated system and adiabatic system?
Re: Isolated vs. Adiabatic system. The internal energy of an isolated system will not change over time. In an adiabatic system, energy is not transferred as heat, but the internal energy can still change if energy is transferred to or from the system as work.
What is an adiabatic process with an example?
Adiabatic Process Examples
In an engine, gasoline is mixed with air and then adiabatically compressed, causing its temperature to increases rapidly and dramatically. Then, a spark plug ignites the air-gasoline mixture in the cylinder. It then undergoes another adiabatic process as the gas in the cylinder expands.
What is an adiabatic system?
An adiabatic process is defined as a process in which no heat transfer takes place. This does not mean that the temperature is constant, but rather that no heat is transferred into or out from the system
What are the types of adiabatic?
Adiabatic Process - Definition, Equation, Reversible ...
The thermodynamic process in which there is no exchange of heat from the system to its surrounding neither during expansion nor during compression. The adiabatic process can be either reversible or irreversible.
Adiabatic process
Adiabatic process formula
Adiabatic system in thermodynamics pdf
Adiabatic process example
Adiabatic system in thermodynamics notes
Adiabatic system in thermodynamics formula
Adiabatic system in thermodynamics wikipedia
Adiabatic process Derivation
Комментарии
 0:11:27
0:11:27
 0:07:25
0:07:25
 0:04:25
0:04:25
 0:10:37
0:10:37
 0:09:57
0:09:57
 0:02:53
0:02:53
 0:12:48
0:12:48
 0:04:02
0:04:02
 0:00:56
0:00:56
 0:00:22
0:00:22
 0:20:41
0:20:41
 1:21:53
1:21:53
 0:07:06
0:07:06
 1:39:10
1:39:10
 0:01:20
0:01:20
 0:40:19
0:40:19
 0:08:37
0:08:37
 0:24:42
0:24:42
 0:04:43
0:04:43
 0:17:42
0:17:42
 1:18:28
1:18:28
 0:10:25
0:10:25
 0:00:36
0:00:36
 0:11:57
0:11:57