filmov
tv
Ideal Gas Law: Total Pressure of Two Flasks
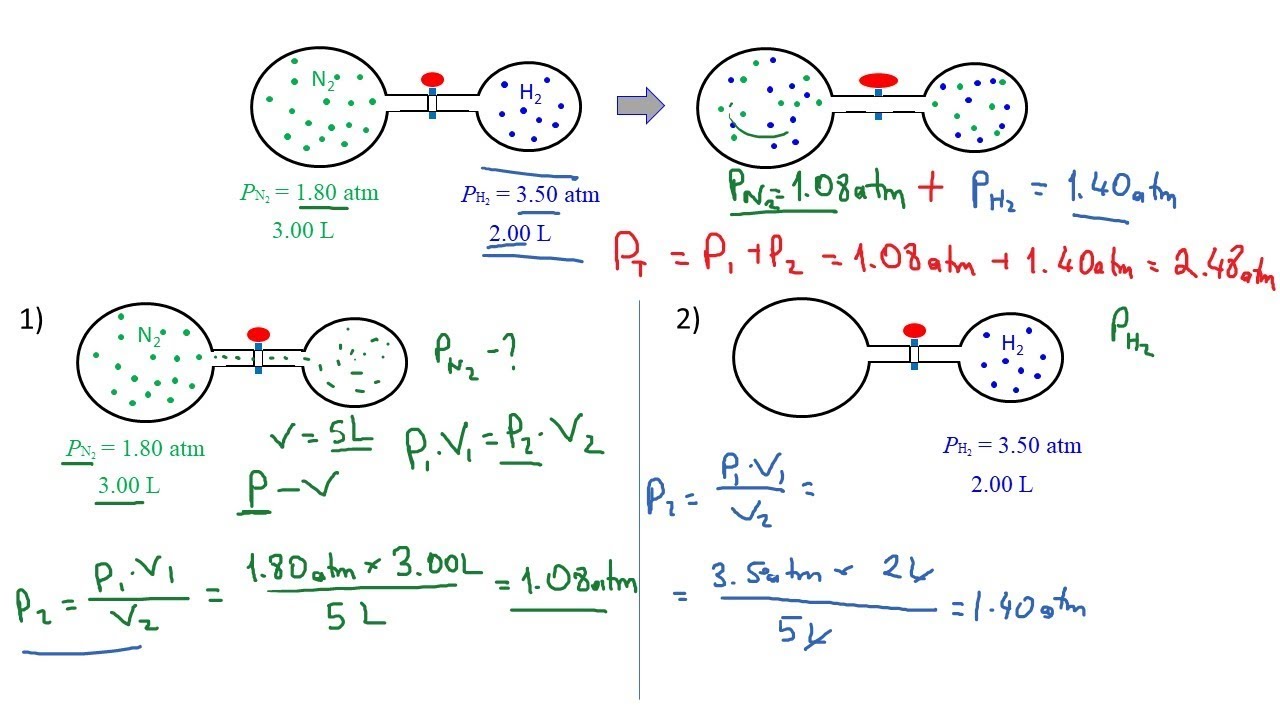
Показать описание
To find more practice problems on gases, other topics, and general chemistry study guides, visit
Ideal Gas Law: Total Pressure of Two Flasks
Dalton's Law and Partial Pressures
Partial Pressures & Vapor Pressure: Crash Course Chemistry #15
Daltons Law | Partial Pressures
Gas mixtures and partial pressures | AP Chemistry | Khan Academy
Gas Law Formulas and Equations - College Chemistry Study Guide
Dalton's Law of Partial Pressure Problems, Mole Fraction, Chemistry Gas Laws
Kinetic Molecular Theory and the Ideal Gas Laws
9 4 The Ideal Gas Law and Partial Pressures
General Chemistry II - Ideal Gas Law - Determining Final Pressure in a Chemical Reaction
⚗️ Total Pressure and Partial Pressures (Question 2)
Ideal Gas Law and Applications
The ideal gas law
How to Calculate Partial Pressure and Total Pressure Using Dalton's Law Examples and Problems
Total Pressure of Gas Mix, Gas Density, Molar Mass of Gas Lecture
Partial pressure and the ideal gas law
General Chemistry - Properties of Gases - Partial Pressures
Lesson 4: Ideal Gas Law Variations and Partial Pressures
Gas Mixtures and Dalton's Law of Partial Pressures
⚗️ Total Pressure and Partial Pressures (Question 1)
Ideal Gas Law, Mole Fraction, Partial Pressure
Gas Laws | Dalton's Law of Partial Pressure | Chemistry Tutorial
Ideal Gas Law
Solving Ideal Gas Law Problems | Calculate Number of Moles (Part 2, Q1)
Комментарии
 0:05:02
0:05:02
 0:06:37
0:06:37
 0:11:55
0:11:55
 0:07:05
0:07:05
 0:06:23
0:06:23
 0:19:24
0:19:24
 0:23:15
0:23:15
 0:05:11
0:05:11
 0:21:48
0:21:48
 0:10:05
0:10:05
 0:05:51
0:05:51
 0:10:45
0:10:45
 0:11:43
0:11:43
 0:07:20
0:07:20
 0:39:23
0:39:23
 0:11:42
0:11:42
 0:07:35
0:07:35
 0:45:33
0:45:33
 0:05:30
0:05:30
 0:06:26
0:06:26
 0:23:38
0:23:38
 0:08:36
0:08:36
 0:05:53
0:05:53
 0:04:49
0:04:49