filmov
tv
How to Draw the Lewis Dot Structure for N 3- (Nitride ion)

Показать описание
A step-by-step explanation of how to draw the N3- Lewis Dot Structure.
For the N3- Lewis structure use the periodic table to find the total number of valence electrons for the N atom. Once we know how many valence electrons there are in N we can distribute them around the central atom with the goal of filling the outer shells of each atom.
Because the Nitride ion (N3-) has an extra electron (the negative sign denotes an extra electron) we need to add that to the 5 valence electrons for the neutral atom N.
In the Lewis structure of N3- structure there are a total of 8 valence electrons. N3- is also called Nitride ion.
----- Lewis Resources -----
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
For the N3- Lewis structure use the periodic table to find the total number of valence electrons for the N atom. Once we know how many valence electrons there are in N we can distribute them around the central atom with the goal of filling the outer shells of each atom.
Because the Nitride ion (N3-) has an extra electron (the negative sign denotes an extra electron) we need to add that to the 5 valence electrons for the neutral atom N.
In the Lewis structure of N3- structure there are a total of 8 valence electrons. N3- is also called Nitride ion.
----- Lewis Resources -----
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
How To Draw Lewis Structures
Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures
Lewis Dot Structures
How to Draw Lewis Structures: Five Easy Steps
Organic Chemistry - How To Draw Lewis Structures
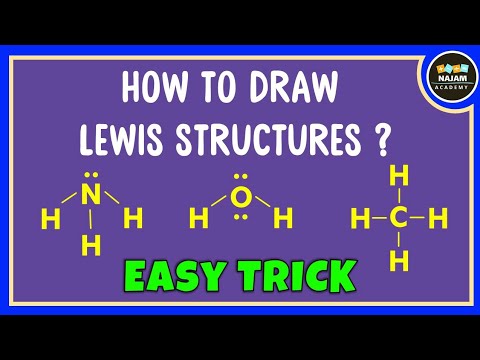
How to draw Lewis structure of any compound? Easy Trick
How To Draw The Lewis Structures of Ionic Compounds
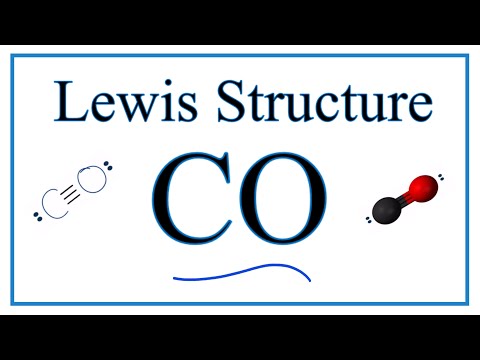
How to Draw the Lewis Dot Diagram for Carbon monoxide (CO)
Lewis Hamilton ||how to draw Lewis Hamilton 🏎️|| #shorts #f1shorts #lewishamilton
How to Draw Lewis Structures for Organic Chemistry
Drawing Lewis diagrams | AP Chemistry | Khan Academy
Lewis Structures Made Easy: Examples and Tricks for Drawing Lewis Dot Diagrams of Molecules
How to Draw Lewis Structures, The Octet Rule and Exceptions | Study Chemistry With Us
Lewis Structures for Covalent Molecules: Step-by-Step
Quick & Easy: 5 Steps to Drawing Lewis Structures with Examples, Practice Problems, Summary, Exp...
How to Draw Lewis Structures and Calculate Formal Charge
How to Draw the Lewis Dot Structure for Carbon monoxide
NO3- Lewis Structure: How to Draw the Lewis Structure for NO3-
Lewis Structure | Trick to Draw Lewis Dot Structures
How to Draw Lewis Structure SHORTCUT: Super Quick and Easy Way with Many Examples
Lewis Diagrams of Ions Made Easy
Drawing Lewis Structures Checklist For Organic Chemistry
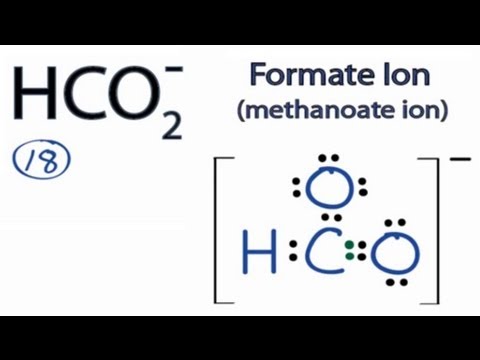
HCO2- Lewis Structure: How to Draw the Lewis Structure for HCO2-
NH2- Lewis Structure: How to Draw the Lewis Structure for NH2-
Комментарии
 0:11:50
0:11:50
 0:07:26
0:07:26
 0:04:41
0:04:41
 0:05:57
0:05:57
 0:11:55
0:11:55
 0:06:19
0:06:19
 0:06:23
0:06:23
 0:02:33
0:02:33
 0:00:12
0:00:12
 0:10:36
0:10:36
 0:07:08
0:07:08
 0:11:57
0:11:57
 0:36:27
0:36:27
 0:10:29
0:10:29
 0:08:42
0:08:42
 0:09:39
0:09:39
 0:01:20
0:01:20
 0:01:59
0:01:59
 0:22:42
0:22:42
 0:07:32
0:07:32
 0:07:56
0:07:56
 0:01:00
0:01:00
 0:01:46
0:01:46
 0:00:58
0:00:58