filmov
tv
Equilibrium Constant Kp | A level Chemistry | Explained

Показать описание
Equilibrium Constant, Kp.
A level Chemistry Explanation Video.
Other related Videos:
Equilibrium & Le chatelier:
Equilibrium Constant Kc:
00:05 Intro
00:31 Dynamic equilibrium
01:53 Equilibrium Constant
02:53 Kp vs Kc
03:53 Partial Pressure
05:49 Kp Expression
09:13 Units of Kp
12:03 Calculating Kp 1
13:05 Calculating Partial Pressure
16:17 Le Chatelier's Principle & Kp
20:51 Calculating Kp 2
25:41 Different Kp calculations
29:46 Kp advice and tips
A level Chemistry Explanation Video.
Other related Videos:
Equilibrium & Le chatelier:
Equilibrium Constant Kc:
00:05 Intro
00:31 Dynamic equilibrium
01:53 Equilibrium Constant
02:53 Kp vs Kc
03:53 Partial Pressure
05:49 Kp Expression
09:13 Units of Kp
12:03 Calculating Kp 1
13:05 Calculating Partial Pressure
16:17 Le Chatelier's Principle & Kp
20:51 Calculating Kp 2
25:41 Different Kp calculations
29:46 Kp advice and tips
Equilibrium Constant: Kp | A-level Chemistry | OCR, AQA, Edexcel
Equilibrium Constant Kp | A level Chemistry | Explained
Kp, Equilibrium Constant (A-level IB Chemistry)
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
AQA 1.10 Equilibrium Constant Kp
AQA A-Level Chemistry - Equilibrium Constant, Kp
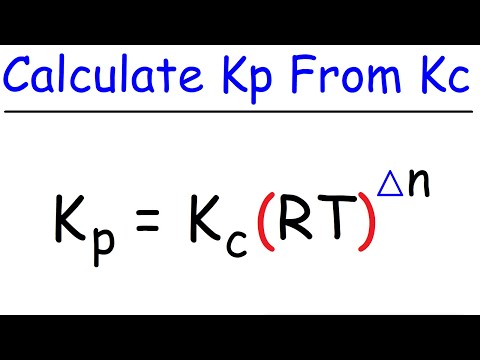
How To Calculate Kp From Kc - Chemical Equilibrium
The Equilibrium Constant Kp
11/Q.P-1/TN 11th/2025/Q. paper Series/Chemistry
Equilbrium Constant Expression Kp
Chemical Equilibria and Reaction Quotients
Equilibrium constant (Kp) - A-Level Chemistry Key Terms #alevelchemistry #chemistryrevision
Quick Revision - The equilibrium constant Kp
Kp, Equilibrium Constant | A level Chemistry | Question Walkthrough
Understanding The Equilibrium Constant, Kp (A2 Chemistry)
Equilibrium constant Kp By Dr Bbosa Science
The Equilibrium Constant
Kp: The Equilibrium Constant | A-Level Chemistry
Kp - Gas Equilibria
Equilibrium constant, Kp - A-level chemistry question walk through
The equilibrium constant Kp Writing expressions and calculating Kp
Kp | Equilibrium Constant | Exam Question Walkthrough | A level Chemistry
Worked examples: Calculating equilibrium constants | Equilibrium | AP Chemistry | Khan Academy
Equilibrium Constant Kp - Past Paper Exam Question Walkthrough|A Level Chemistry (AQA)
Комментарии
 0:16:26
0:16:26
 0:34:23
0:34:23
 0:13:09
0:13:09
 0:53:22
0:53:22
 0:22:23
0:22:23
 0:30:46
0:30:46
 0:10:51
0:10:51
 0:10:40
0:10:40
 0:11:13
0:11:13
 0:03:04
0:03:04
 0:06:48
0:06:48
 0:00:14
0:00:14
 0:09:05
0:09:05
 0:10:20
0:10:20
 0:20:26
0:20:26
 0:12:24
0:12:24
 0:06:15
0:06:15
 0:07:29
0:07:29
 0:04:41
0:04:41
 0:04:12
0:04:12
 0:17:53
0:17:53
 0:10:51
0:10:51
 0:08:12
0:08:12
 0:21:53
0:21:53