filmov
tv
How to find the Oxidation Number for C in CO2 (Carbon dioxide)

Показать описание
To find the correct oxidations number for CO2 (Carbon dioxide), and each element in the molecule, we use a few rules and some simple math.
First, since the CO2 molecule doesn’t have an overall charge (like NO3- or H3O+) we could say that the total of the oxidation numbers for CO2 will be zero since it is a neutral molecule.
We write the oxidation number (O.N.) for elements that we know and use these to figure out oxidation number for C.
----------
GENERAL RULES
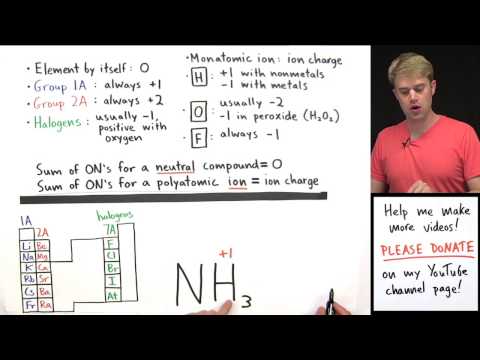
In a netural molecule all Oxidation numbers must add up to zero.
Group 1 = +1
Group 2 = +2
Hydrogen with Non-Metals = +1
Hydrogen with Metals (or Boron) = -1
Fluorine = -1
Oxygen = -2 (except in H2O2 or with Fluorine)
Group 17(7A) = -1 except with Oxygen and other halogens lower in the group
----------
We know that Oxygen usually is -2 with a few exceptions. When Oxygen is in a peroxide, like H2O2 (Hydrogen peroxide), it has a charge of -1. When it is bonded to Fluorine (F) it has an oxidation number of +2.
Here it is bonded to element symbol so the oxidation number on Oxygen is -2. Using this information we can figure out the oxidation number for the element C in CO2.
First, since the CO2 molecule doesn’t have an overall charge (like NO3- or H3O+) we could say that the total of the oxidation numbers for CO2 will be zero since it is a neutral molecule.
We write the oxidation number (O.N.) for elements that we know and use these to figure out oxidation number for C.
----------
GENERAL RULES
In a netural molecule all Oxidation numbers must add up to zero.
Group 1 = +1
Group 2 = +2
Hydrogen with Non-Metals = +1
Hydrogen with Metals (or Boron) = -1
Fluorine = -1
Oxygen = -2 (except in H2O2 or with Fluorine)
Group 17(7A) = -1 except with Oxygen and other halogens lower in the group
----------
We know that Oxygen usually is -2 with a few exceptions. When Oxygen is in a peroxide, like H2O2 (Hydrogen peroxide), it has a charge of -1. When it is bonded to Fluorine (F) it has an oxidation number of +2.
Here it is bonded to element symbol so the oxidation number on Oxygen is -2. Using this information we can figure out the oxidation number for the element C in CO2.
Комментарии
 0:31:15
0:31:15
 0:07:00
0:07:00
 0:13:26
0:13:26
 0:16:05
0:16:05
 0:13:13
0:13:13
 0:00:56
0:00:56
 0:04:27
0:04:27
 0:15:25
0:15:25
 0:38:43
0:38:43
 0:06:00
0:06:00
 0:00:25
0:00:25
 0:04:11
0:04:11
 0:01:28
0:01:28
 0:03:56
0:03:56
 1:06:37
1:06:37
 0:04:58
0:04:58
 0:09:47
0:09:47
 0:01:35
0:01:35
 0:03:19
0:03:19
 0:03:52
0:03:52
 0:01:34
0:01:34
 0:01:07
0:01:07
 0:01:46
0:01:46
 0:06:45
0:06:45